|
Chemguide: Support for CIE A level Chemistry Learning outcome 11.3(g) This statement is about using resources more efficiently in terms of materials, energy and the environment. Before you go on, you should find and read the statement in your copy of the syllabus. A list of Twelve Principles of Green Chemistry was published in 1998. The list below is my translation of the original list into slightly easier language. You do not need to learn all these. What the list might do is to give you ideas for answers to some of CIE's slightly vague questions that they seem to ask in this section of the syllabus. The twelve principles are:
This is all just common sense. It makes sense economically, and also environmentally. Questions on statement 11.3(g) could involve material from right across the whole syllabus as well as any new information you might be given. One question (June 2010 papers 42 or 43 Q8), for example, was about developments in batteries, but there was nothing in it that you couldn't answer from previous knowledge or information in the question. You did, though, need to be able to calculate a cell potential from information from the Data Booklet. I want to look at one topic that had come up twice up to the June 2011 exam session, and then various other bits and pieces from questions or the "What the student needs to know" section from the second version of the Applications Support Booklet. Using bacteria to recover metals from low-grade sources The two questions about this concerned the extraction of copper from low grade ores or from mine waste using bacteria. You were given all the information you needed to understand what was going on. There are bacteria which have the ability to oxidise iron(II) ions to iron(III) ions, and sulphide ions into sulphuric acid. These products (the iron(III) ions and sulphuric acid) can convert highly insoluble copper ores into solutions of copper(II) sulphate. Copper occurs in lots of different ores including
. . . and lots more. The most important ore of copper is chalcopyrite - and you can see that this contains both iron(II) ions and sulphide ions as well as copper(II) ions. If you want to remember a copper ore, remember this one. The sulphuric acid produced will react with all of these ores to make copper(II) sulphate solution. | |
|
Note: If you are really awake, you might wonder how the copper(I) sulphide in chalcocite ends up as copper(II) sulphate. That's where the iron(III) ions that the bacteria produce play a part. Iron(III) ions are good enough oxidising agents to oxidise copper(I) ions to copper(II) ions. | |
|
So the result is a very dilute solution of copper(II) sulphate. To extract the copper, the solution is concentrated and then electrolysed to produce copper at the cathode. The CIE questions were happy to extract the copper using a more reactive metal such as iron to give iron(II) sulphate and copper. This is unlikely to be done industrially. The questions also asked some things involving common sense. They asked, for example, what advantages and disadvantages there were in this method. Advantages might include that it doesn't need much investment in machinery or labour, it doesn't need much energy, and it is very quiet. Disadvantages include the fact that it is very slow. Energy sources Fuel cells Fuel cells were dealt with in learning outcome 6(j). You need to go back and revise this. There is nothing much to add to this, apart from considering some advantages and disadvantages of using fuel cells like this in motor vehicles. The obvious advantage is that the only product from the reaction is water. Traffic exhaust pollution is therefore no longer a problem. The disadvantages include the need to store hydrogen in the vehicle safely, and the costs (economic and environmental) of producing the hydrogen in the first place. Hydrogen can be produced by electrolysis, but you need to generate the electricity to do that. The Applications Support Booklet suggests that you only get half the energy you used to produce the hydrogen back when the hydrogen reacts in a fuel cell. That doesn't seem to be a sensible way of doing things. Hydrogen can also be produced from natural gas, but that is a diminishing resource, and the process also produces carbon dioxide. Biofuels This includes bioethanol and biodiesel. There was a full question about biofuels in the specimen paper for the current syllabus. This is easily available from the CIE website. If you haven't already got them, download both the question paper and mark scheme for paper 4 from the specimen papers list. There is nothing difficult about this apart from the fact that it assumes you know the formula for cellulose in part (c) of Q10. Look it up in the mark scheme, and learn it. Look carefully at the mark scheme for part (d) as well. Notice that there are 3 marks for the question. That means that you have to think of three things to say about it, each of which deserves a mark. It didn't come up as a part of this question, but you also need to think of some disadvantages of using biofuels. One fairly obvious one is that growing biofuels needs land which might otherwise be used to produce food crops. Food crops which are grown for fuel can't feed people. If something gets into short supply, its price rises. There is good evidence that a big effort to produce more biofuels in the US in the late 2000s caused a surge in world grain prices. The example of bagasse in the specimen question gets around this by using parts of the crop which would otherwise go to waste. And another thought . . . Biofuels are sometimes described as carbon neutral. They take in carbon dioxide from the air when they are growing, and release it back again when they are burnt to produce energy. But that disregards the amount of carbon dioxide that may have been produced in making and transporting fertiliser, in sowing and harvesting the crop, in processing the crop into fuel, and in transporting the fuel to wherever it is needed. Things are rarely as straightforward as they seem! Other energy sources In the "What the student needs to know" section of the second version of the Application Support Booklet it says that you should know some of the arguments for or against nuclear power. I simply can't see that that has anything to do with the syllabus statement which asks for everything to be discussed in terms of a knowledge of chemistry. There is nothing relating to a knowledge of chemistry in the pros and cons of nuclear power. So I'm not going to talk about this! It hasn't been asked up to the June 2013 exam, and I am sure that you can think of a few reasons for and against nuclear power. The same thing applies to other energy sources such as hydroelectric energy, wind energy or solar energy. Recycling Up to June 2013, CIE have asked only one question about recycling, worth 3 marks. It asked you to decide what the reasons were for recycling glass, steel and plastics. You had to decide whether they were recycled to save energy or save resources, and explain your choices. You obviously need to know something about the raw materials and manufacturing processes of the things which are commonly recycled. Glass is made mainly from silicon dioxide. The silicon dioxide comes from sand - not a scarce resource! Making glass uses lots of energy; recycling glass saves that energy. Steel (a form of iron) is made from iron ores which are reasonably plentiful, but making it uses lots of energy, and mining the ore is also energy-intensive. The main reason is therefore to save energy. However, CIE allowed you the mark for saving a resource on the grounds that iron ore is getting used up. So you could hardly lose with this bit of the question. Plastics are recycled because they come from crude oil - a valuable and finite resource. Aluminium (not asked in the question, but it could have been) is recycled because extracting it from its ore uses huge amounts of electricity. Paper (not asked so far, and I'm not very convinced there is any chemistry involved - so it probably won't be asked) is mainly recycled to save energy. There is a discussion about it on this Wikipedia page. Just read the first few paragraphs, and don't waste too much time on it. Two unusual solvents Supercritical carbon dioxide Every gas has a critical point - a temperature and a pressure above which you can't change the gas into a liquid by compressing it. Carbon dioxide's critical point is at 31°C and 73 atmospheres. Supercritical carbon dioxide is where the gas is at higher temperatures or pressures than this. You can think of it as having properties of both a gas and a liquid. It is being used as a solvent in all sorts of different situations - usually to extract one material from a mixture. For example:
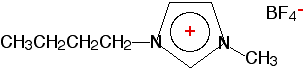
In each case, supercritical carbon dioxide can replace much more hazardous solvents. It is also easy to separate the carbon dioxide from the solution of the extracted material - you can just lower the pressure, and it will come off as a gas which can be collected and converted back into supercritical carbon dioxide again. Ionic liquids Molten sodium chloride is an ionic liquid, but that isn't what we are discussing here. What we are interested in are room temperature ionic liquids. You know, of course, that attractions between ions are very strong, and this gives ionic compounds high melting points. So how can any ionic compounds exist as liquids at room temperature? Note that we aren't talking about solutions of ionic compounds - we are talking about pure ionic compounds. This can happen if one or both of the ions present in the compound is bulky and awkwardly shaped. In that case, the ions find it very difficult to come together to produce a stable lattice. There are several combinations of ions that this can happen to. Here is one of them. You don't need to learn it.
You can see that it might well be difficult to fit these ions into a simple lattice. | |
|
Note: In case you are wondering, the circle in the middle of the pentagon of 3 carbon atoms and 2 nitrogen atoms means exactly the same thing as the circle in the middle of a hexagon for benzene. There is delocalisation of charge and electrons over the whole of the pentagon. | |
|
Ionic liquids are excellent solvents for all sorts of different things - ionic and covalent. They can be used to extract oil from oil shales, or to clean up oil spills on beaches. They will also dissolve otherwise insoluble things like copper oxide or uranium oxide. What makes them especially good is that they don't release toxic vapours unlike most organic solvents. The reason for this is that although the ions can't fit together into a stable lattice, there are still strong electrostatic forces between them. That means that ionic liquids are almost entirely non-volatile. That isn't to say that they are completely safe, though. Their aqueous solutions are toxic with unpredictable effects on aquatic life. Ionic liquids are also electrolytes because they contain mobile ions. They may well be involved in the design of new types of batteries. Up to the June 2013 exam, CIE had only asked a single question about ionic liquids, and that was in a question about new batteries. The information in the question said that the battery contained some sort of paper soaked in an ionic liquid electrolyte. A question (worth 1 mark) asked you to name an example of an ionic liquid electrolyte which wasn't a solution. You don't need to know anything about ionic liquids (in the sense that we have been talking about them) to answer this. Any molten electrolyte will do - molten sodium chloride, for example. That is because the question doesn't ask for an ionic liquid which could be used in a battery - just for an ionic liquid. Obviously, you couldn't use molten sodium chloride in any usable battery - it doesn't melt until 801°C. The Examiner's Report was right to criticise the many candidates who suggested ionic solutions, because the questions specifically told you not to use them. People will have told you many times to "read the question" - that's because it matters! Up to this point, then, they have never asked anything about room temperature ionic liquids, but it seems to me to be a topic that it would be easy to build a question around. They could give all the necessary background detail, and then ask things like:
. . . and so on. You don't need to have any knowledge of the process the question is asking about, but you do need to be able to apply knowledge you already have. And finally . . . Whatever order you have been working through the syllabus in, Section 11.3 is likely to be the last section you will do. And this is the last statement in Section 11.3. Congratulations on getting this far without giving up. And good luck when you come to take your exams.
© Jim Clark 2011 (modified August 2013) |
|