|
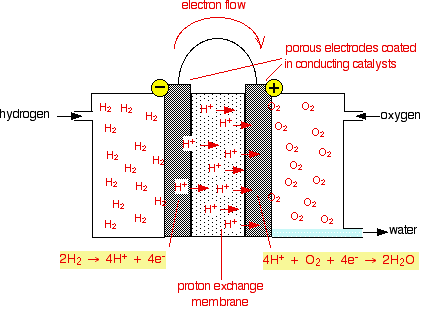
Chemguide: Support for CIE A level Chemistry Learning outcome 6(j) This statement is about fuel cells. Before you go on, you should find and read the statement in your copy of the syllabus. It is very difficult to know exactly what CIE want for this statement, because as far as I can tell it hasn't been covered at all in any question up to November 2012. There was a question which mentioned fuel cells in Paper 41 in the May/June 2010 exam, but it gave a very simplified diagram of an old-fashioned fuel cell, and then asked typical electrode potential questions. Knowing about fuel cells in advance wouldn't have helped at all with the question. I will look at a part of this question further down this page. The teacher support material suggests getting students to do an internet search to find out about current developments. If you do this, you will immediately find that there are lots of different types of fuel cells. A simple fuel cell One of these types is called a Proton Exchange Membrane Fuel Cell (PEMFC), or a Polymer Electrolyte Membrane Fuel Cell (also PEMFC). There is a simplified diagram below:
Essentially, this has two metal electrodes covered with a fine layer of metal catalyst, for example platinum. This catalyses the reactions at the two electrodes. Sandwiched between these is a polymer-based membrane through which protons (hydrogen ions) can diffuse. One electrode is exposed to hydrogen gas; the other to oxygen or air. The catalyst on the electrode exposed to the hydrogen catalyses the reaction:
You may notice that I have doubled that equation on the diagram above. I will explain why in a moment. On the electrode exposed to the oxygen, this happens:
So, overall, at the left-hand electrode, hydrogen molecules ionise to give hydrogen ions and electrons. Those hydrogen ions diffuse through the membrane to the right-hand electrode, where they react with oxygen to make water. The overall reaction in the fuel cell can be found by adding the two equations together, but because of the need for four electrons at the right-hand electrode, the hydrogen equation has to happen twice.
Giving:
Notice that the only waste product from this cell is water. I was going to show you how to use electrode potentials to find the emf of a cell like this, but just realised that you can't do that! Standard electrode potentials relate to ions in solution. In this particular case you don't have solutions - certainly not at the electrode on the hydrogen side. We will look at electrode potentials in the next example instead. CIE May/June 2010 Paper 41 Q5 The question started with a simple diagram of a beaker with sodium hydroxide solution in it. It contained two inert electrodes. Hydrogen gas was blown into the solution over the left-hand electrode; oxygen was blown in over the right-hand one. It told you that the following reactions were taking place: At the left-hand electrode:
At the right-hand electrode:
One of the things that it asked you to do was to find the overall equation for the cell reaction. The Examiner's Report said that this was poorly done by students. I have little sympathy for students who can't get something as simple as this right! All you have to do is multiply the first equation by 2 so that you have the same number of electrons in each, and then add them up. Then cancel out anything which appears on both sides. If you do that, you will end up with the equation:
Back to the more difficult bit: You were also asked to calculate the standard cell potential using the Data Booklet. The first thing you should do is to find the alphabetical list of electrode potentials in the Data Booklet towards the end of the syllabus. Have that on your screen alongside your browser window if possible. Now you need to look for the equilibria and E° values corresponding to the equations you have been given. When you look for them, don't forget that the equation may be reversed in the list. All the equilibria are written with the electrons on the left-hand side. In this case, then, the equilibrium you are looking for with the hydrogen in it will be the reverse of the equation you are given. You should find these two equilibria, with their accompanying E° values:
Now you can calculate the cell potential using the formula: E°cell = E°right - E°left E°cell = +0.40 - (-0.83) volts = +1.23 v The sign shows that the right-hand electrode is positive. That means that electrons will flow through the external circuit from the hydrogen side to the oxygen side. The question continued with questions about what happened if the concentration of the sodium hydroxide solution changed. But this has nothing specifically to do with fuel cells. Then in the second half of the question, it more or less repeated the whole process with what happens in a lead-acid battery. Again, you didn't have to know anything about such batteries - all the information was in the question. This is, however, a poorly set question, because it tests the same skills twice. If you couldn't do the first half of the question, you were penalised all over again in the second half. The Examiner's Report said that this was the lowest scoring question on the paper. I'm not surprised! I suggest you have a careful look at the rest of this question, together with the mark scheme and the Examiner's Report. Other odds and ends The syllabus says that you should be able to state the possible advantages of developing other types of cell, for example the hydrogen-oxygen fuel cell and improved batteries (as in electric vehicles) in terms of smaller size, lower mass and higher voltage. They haven't asked anything specifically on that up to November 2012, and there isn't much you can add to the syllabus statement. The statement tells you all you really need to know. In addition, the teacher support material mentions "the problems with taking a high current from the cell (high internal resistance, and slow equilibration at the gas-electrode interface)." Any single fuel cell can only produce a limited voltage. The maximum possible value (the emf) only applies if no current flows from the cell. The more current you take, the lower the voltage tends to get. This is an effect of the high internal resistance of the cell. In order to produce the sort of voltages and currents you need for applications like transport, you need a whole stack of cells. The comment in the teacher support material about "slow equilibration at the gas-electrode interface" means that even in the presence of the catalytic coating on the electrodes, it takes some time for the equilibria to set up. If you draw a lot of current, the equilibria may not have time to set up properly, and that will effect the voltage that you can get from the cell.
© Jim Clark 2011 (modified August 2013) |