|
Chemguide: Support for CIE A level Chemistry Learning outcome 11.3(e) This statement is about nanotechnology. Before you go on, you should find and read the statement in your copy of the syllabus. The nanoscale If you are going to think about nanoscale particles, you obviously have to have some idea of how big these are. A nanometre (nm) is 10-9 metres. You will be familiar with this as the scale that the radii of atoms are measured on. For example, a sodium atom has a metallic radius of 0.186 nm; a carbon atom has a covalent radius of 0.077 nm. The usual sizes of molecules range from about 0.1 nm to 10 nm. Obviously there are much bigger ones as well. In June 2011, CIE set a question asking specifically for the range of sizes of nanoparticles on a scale labelled from 10-6 to 10-12 m. The mark scheme allowed answers for the smallest nanoparticles of between 10-8 and 10-9, and the biggest ones from 10-6 and 10-7. Turning that into nm, they are suggesting that nanoparticles fall into the range from about 1 nm up to about 1000 nm. A more usually quoted range is from 1 to 100 nm. Remember that. A similar question was asked in June 2012. If you need to convert it into metres, then 1 nm is 10-9 m; 100 nm is 100 x 10-9 m or 10-7 m. But it is much easier to remember 1 - 100 nm, and then convert it if you need to. Nanoparticles on a biological scale Another CIE question asked you to put the relative sizes of the length of a strand of DNA, a nanoparticle, and a cell in order of size. The tricky bit here is the length of a stretched out bit of DNA. I have to admit that I had absolutely no idea what that was! Although DNA fits inside cells, this is because it is coiled up. If you stretch it out, a length of human DNA can be up to about 5 cm long. That means that the smallest of the things you are supposed to be comparing is the nanoparticle, next the cell, and then DNA is the longest. | |
|
Note: Human DNA can be up to about 150,000,000 base pairs long. Apparently 3 base pairs is about 1 nm in length. That means that the DNA length will be 50,000,000 nm, which works out at 5 cm. In the Examiner's Report, CIE said that few candidates could do this question. I am not in the least bit surprised. | |
|
Buckminsterfullerene and other carbon-based nanoparticles You almost certainly know about the two main allotropes of carbon - diamond and graphite. And you should also be able to define the word "allotrope". Allotropes are two or more different forms of an element in the same physical state. In a CIE question, they wanted you to go a bit further than this by saying that this was because the different allotropes had different arrangements of the atoms. So learn this:
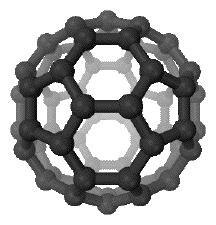
In 1985, a third allotrope of carbon was discovered, known as buckminsterfullerene or buckyballs. Buckminsterfullerene Buckminsterfullerene, C60, is a carbon molecule made of 60 atoms arranged like the faces of a soccer ball - in pentagons and hexagons. That is why it is commonly known as buckyballs. This is the simplest of a family of similar nanoparticles known as fullerenes.
| |
|
Note: This diagram was taken from Wikipedia. | |
|
An individual buckyball has a diameter of about 1 nm. Physical properties of buckyballs I have found researching this fairly confusing, and so will base what I say mainly on information from the Applications Support Booklet and CIE's mark schemes. The following all refer to the most commonly discussed fullerene, C60.
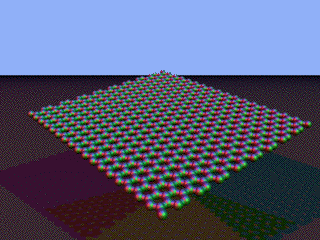
Nanotubes Imagine a single one of the sheets of carbon atoms which make up graphite. This is known as graphene.
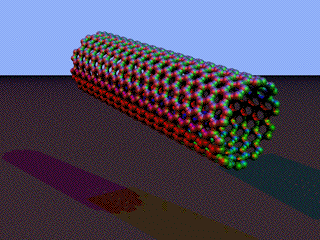
Now imagine that you could roll this up into a tube. Well, you can - and it gives you a carbon nanotube.
| |
|
Note: These images were adapted from this Wikipedia page. | |
|
These tubes have a diameter of about 1 nm, but can be millions of times longer. Individual nanotubes are the strongest and stiffest things yet discovered (according to Wikipedia) because of the very strong forces of attraction between the atoms making up the tubes. However, attractions between individual tubes will be weaker, and so bulk material made of carbon nanotubes won't be quite so strong because of the weaker forces between the tubes. CIE asked a question (November 2009 paper 41 Q8) about the use of nanotubes in making light, flexible electrical batteries. You didn't need to know anything about this application of nanotubes before, but you did need to know what a nanotube was and how it was produced. The question involved having a paper-like material which was coated in carbon nanotubes. One of the questions that was asked wanted to know how the paper could be rolled up despite the nanotubes being so stiff. The simple answer (not easy to think of in an exam) was just one of scale. The nanotubes coated on to the paper were at the nano scale. You didn't have single tubes running the whole length of the paper. In order to curve the paper, all that needs to happen is for the tubes to move relative to each other. You don't need to break the tubes. Some other important properties:
Uses of nanotubes There are a large number of uses for carbon nanotubes, and you might like to have a look at some of them on this page from UnderstandingNano.com. You could follow up some links from that page if you are interested, but don't think that you have to learn any of it. Any questions that are asked about this should give you any extra knowledge you need. Basically, you need to know what nanotubes are, and that nanotubes are very strong, very light, and can conduct electricity. There are so many potential uses of this material that it is completely impossible to try to guess what questions CIE might come up with in the future. Nano test-tubes If you have a short carbon nanotube and attach half a buckyball to one end, you have created a miniature test-tube. That is really all you need to know! Nanoparticles and light Two examples of this have come up in exam papers up to June 2013. One concerned the use of nano-sized particles of titanium dioxide used in sunscreens. You were asked how these particles can protect you from harmful UV radiation. The answer was simply that the nanoparticles are similar in size to the wavelength of UV light. That means that the light is reflected back or scattered by the nanoparticles. It is hard to see how you could actually deduce this unless you knew a reasonable amount of physics. The Examiner's Report said that most candidates got the mark for reflection of the light, but few could give a reason. That, again, doesn't surprise me. What wasn't mentioned in this question, but might have been, was the worry some people have that because these nanoparticles are so small, they could pass through the skin into the body with unpredictable consequences. The other question asked about nano-particle coatings on windows which reflect heat, but let visible light through. This was another poorly scoring question, which the Examiner's Report suggest was "easy marks". It is only easy marks if you know quite a lot of physics. Apart from anything else you have to recognise that in electromagnetic radiation, the infra-red corresponds to heat energy. If you recognise this, you could reasonably work out that the infra-red (heat) is being reflected back by the coating while the visible light is passing through. The reason for that is that the longer wavelength infra-red light is reflected by the nano-particles, whereas the shorter wavelength visible light gets through the gaps between them. This isn't chemistry. Nanoparticles and drug delivery Drug delivery has already been covered in statement 11.3(b). All you really need to know is that nanoparticles (like hydrogels) can have drugs enclosed within them or attached to them. They are also small enough to pass through cell membranes into the cells themselves. The blood vessels of tumours have rather larger pore sizes than normal blood vessels, and it is easier for nanoparticles to get through these to the cancer cells themselves. This helps the targeting of the drugs to where they are needed. If you are interested, there is a lot more about this on another UnderstandingNano.com page. Once again, you do not need to learn any of this - there is just too much of it! | |
|
Note: Here is a link to the front page of the UnderstandingNano.com site. If you have the time, it might be worth spending a while just reading bits that seem interesting to you. It seems to me that it would be a wonderful source of ideas for a CIE examiner setting questions about nanotechnology. You might strike lucky! The only disadvantage is that the site is badly cluttered up with ads. | |
© Jim Clark 2011 (modified August 2013) |
|