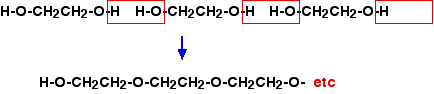|
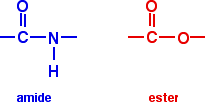
Chemguide: Support for CIE A level Chemistry Learning outcome 11.3(b) This statement is about drug delivery - getting medicinal drugs to where they are needed in the body. Before you go on, you should find and read the statement in your copy of the syllabus. Background There is much better evidence for what CIE expect in this topic than in the previous one about the design of drugs. Up to the time of writing (August 2013), this had been asked four times - either as a full question or a major part of a question. Giving drugs by mouth or by injection There are various reasons why it can be better to inject drugs directly into the blood rather than take them by mouth. You get a faster response, the patient doesn't have to be conscious, and you can use smaller doses. The reason for the use of smaller doses is that the drug doesn't have to find its way through hostile environments such as the acidity of the stomach before it can be absorbed into the blood. Damage to the drug molecules at very low pH is the main reason why it is better to give some drugs by injection. There are two common groups occurring in drugs which are hydrolysed under acidic conditions - esters and amides. It is important that you can recognise these groups in a skeletal structure for a drug. You could expect to be asked to circle two different groups in a drug molecule which could be hydrolysed in the digestive system. One of those drugs was Taxol, and I will use that as an example because I already have a suitable diagram. Taxol has one amide group and four ester groups. Remember that amide and ester groups look like this:
Before you read on, find them in the Taxol structure:
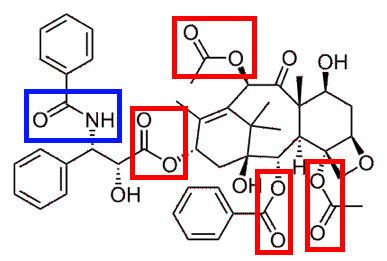
Although CIE actually asked you to draw a circle around each sort of group, in their mark scheme they actually showed particular bonds being circled. They did, though, say that whole groups being circled was allowable. To my way of thinking, if a questions asks you to circle a group, then that is exactly what you should do - otherwise you aren't answering the question, and risk losing marks. It is actually easier to identify and circle the group anyway, because you don't have to worry about exactly which bond in the group is being broken. (Update August 2013: A more recent question asked you to circle bonds. Read the question carefully, and give them exactly what they ask for!)
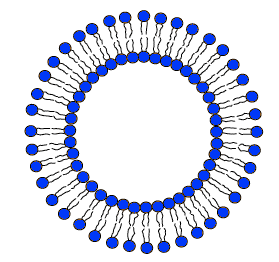
The amide group is shown in blue and the ester group in red. In the exam, you were only asked for one of the ester groups. Please don't try to remember this example! There must be many, many drugs which contain these groups and which the examiners could use in future questions. If I was setting exam questions, I certainly wouldn't re-use Taxol for years and years, if at all. What you should do is to make sure that you understand that amide and ester groups are vulnerable to the acid conditions of the stomach, and be able to recognise them in a drug's structure. Liposomes, hydrogels and PEG These are all involved in ways of getting a drug to where it is needed without damage. Liposomes A liposome is like a little bubble which can carry drugs around. The outside of the bubble consists of a double layer of molecules known as phospholipids. Each of these looks rather like a tadpole with two tails. The diagram shows a liposome. This will have water, or an aqueous solution, around it and inside it.
The head of each molecule contains ionic groups which are attracted to water molecules. They are said to be hydrophilic (literally "water loving"). The tails are hydrocarbon chains. These aren't attracted to water molecules - they are said to be hydrophobic (literally "water fearing"). In an aqueous solution, these molecules arrange themselves in the two layers with the hydrocarbon tails pointing towards each other. This produces the greatest number of attractions. The heads of the molecules are all attracted towards water molecules in the solutions inside and outside the liposome. The tails, of course, are attracted to each other by van der Waals dispersion forces. Nothing is pointing at something it doesn't find attractive. If the liposome forms in an aqueous solution containing a drug, some of the drug will get trapped in the middle of the liposome. If you have a fat-soluble drug, this can get trapped in the layer of hydrocarbon tails. So liposomes are capable of carrying either water-soluble or fat-soluble drugs, and can protect the drug from acidic conditions or attack by enzymes until it is released. The drugs are finally released when the liposomes interact with cell membranes. Hydrogels These came up as a part of a CIE question (June 2008 paper 4 Q 10) - you weren't expected to know anything about them before. The ones in the question were rather like liposomes except that the drug was held in a thick-walled bubble made from a network of polymer chains. You were told that hydrogels swell and absorb water at a rate depending on the pH of the solution around them. As the hydrogel swells, it releases the drug. There were several questions relating to this, but there was one that I thought was perhaps awkward if you hadn't met it before. They asked for two ways in which the hydrogel could be modified to change the rate of drug release. The mark scheme suggested that you could use hydrogels of various thicknesses. Obviously the thicker the material of the "bubble", the more slowly it would release the drug. Otherwise, you could change the composition of the polymer to make it more resistant or less resistant to breaking down. Or you could make a bubble with holes or pores in its walls. Changing the thickness of the bubble wall is fairly obvious. I didn't feel that it was quite as obvious that you could change the material or somehow design holes into it. This question may well never come up again, but looking at it in detail might give you hints about how to answer future similar questions. The only way you can get these sorts of useful ideas is by studying past papers, mark schemes and examiner's reports. There is no short cut to this. PEG PEG is the abbreviation for polyethylene glycol. That needs some explanation. Ethylene glycol is the old name for ethane-1,2-diol, HO-CH2CH2-OH. PEG is a (generally short) polymer based on this. You can work out the structure of PEG like this.
| |
|
Note: This diagram is only to show how you could work out the structure of PEG if you needed to. It isn't the way it is made. The polymer is actually made from epoxyethane in the presence of ethan-1,2-diol. The name PEG is therefore rather misleading. Presumably there isn't any simple way of eliminating water molecules from ethane-1,2-diol as shown in the diagram. The reaction is like the reaction of epoxyethane with alcohols described towards the bottom of the epoxyethane page of Chemguide. There is no indication from the syllabus or the Applications Support Booklet that you need to know this. | |
|
The formula you might find in an exam question would look like this:
That means that there is an -OH group at either end of the polymer molecule. That is important, because it is where drug molecules can get attached. Each PEG molecule can carry two drug molecules. Modern techniques actually modify the -OH groups so that they can react with suitable groups in the drug. You won't be expected to know about this. There have been three questions involving PEG up to the June 2013 exam, all of them straightforward. You could, for example, be asked where a drug molecule would attach to the PEG molecule. All you needed to say was "at one of the -OH groups". Or you could be asked what sort of bonds the PEG part of the molecule carrying the drug might form with other molecules in the digestive system. That could be either hydrogen bonds (from the lone pairs on the oxygens along the chain) or van der Waals attractions. So why would you actually want to attach a drug to such a molecule? Obviously, it isn't going to help to protect a drug from the acidity of the stomach. Amide or ester groups in the drug will still get hydrolysed. PEG is very soluble in water, and attaching a drug to it can increase the solubility of that drug. Attaching a drug to PEG also creates a bigger molecule which is excreted from the body more slowly. A particular dose of drug is therefore more long-lasting. Questions you might get asked Questions where you need some knowledge of chemistry Make sure you know about:
Non-chemistry questions These are questions which rely on general knowledge or common sense. For example, you could be asked:
In addition, there are a whole lot of questions that could be asked about new situations that you might be given. The example of hydrogels I mentioned above is one of these. It is hard to predict every single question that you could be asked in cases like this. Some things you will have to think out for yourself in the exam!
© Jim Clark 2011 (modified August 2013) |
|