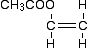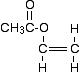|
Chemguide: Support for CIE A level Chemistry Learning outcomes 10.8(d), 10.8(e), 10.8(f) and 10.8(g) These statements are all about the relationships between the structures of monomers and the polymers they form. You must expect that the majority of questions relating to these statements will contain examples that you haven't thought about before. Before you go on, you should find and read the statement in your copy of the syllabus. Statements 10.8(d) and 10.8(f) These are fairly simple, and ask you to predict either:
Predictions about addition polymerisation Starting from the monomer(s) If your monomer contains carbon-carbon double bonds, then it will undergo addition polymerisation. It is possible that you might be given two different compounds containing carbon-carbon double bonds as a pair of monomers. These are known as copolymers. They will still undergo addition polymerisation, but the polymer will obviously be more complicated than if you polymerised just ethene, for example. We will look at this some more in a minute. Copolymers aren't mentioned anywhere in the syllabus, but are discussed in the teacher support material. Starting from the polymer If the main chain of the polymer contains only carbon atoms, then it must be an addition polymer. Predictions about condensation polymerisation Starting from the monomer(s) If the monomer or monomers don't contain carbon-carbon double bonds, then it is condensation polymerisation. Look for groups that you know are involved in this. These could be -OH and -COOH or -COCl for polyesters. Or they could be -NH2 and -COOH or -COCl for polyamides. Starting from the polymer If the main chain of the polymer contains nitrogen or oxygen atoms, then it must be a condensation polymer. Statement 10.8(e) This statement asks you to be able to draw the repeating unit in a polymer from given monomers. For addition polymerisation If you have a single monomer This example is not for you to learn. What is important is that you can understand how to come up with the right answer if a similar - but not the same - example is asked. PVA is used widely as a glue. It's name is based on polyvinyl acetate, and the old name for its monomer is vinyl acetate, with the formula CH3COOCH=CH2. So what is the repeating unit in PVA? Because of the carbon-carbon double bond, we are looking at addition polymerisation. The first thing to do is to redraw the formula with the emphasis on the carbon-carbon double bond, like this:
Or, perhaps better, because it shows the structure better:
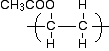
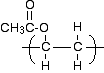
You would have hints from the way the examiners had drawn the monomer as to which they probably wanted. If in doubt, give as much detail as you can, but don't mess it up by getting any of the detail wrong! Now turn the double bond into a single bond, and draw the bonds showing that the structure continues to the left and right. Then complete it by wrapping the brackets around the repeating unit. Depending on which way you draw the ester group, your final structure would be either
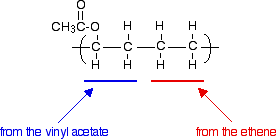
or
You shouldn't find this in any way difficult! If you have more than one monomer You can make a copolymer called EVA, by combining the monomers ethene and vinyl acetate. For exam purposes, you just show the two monomers joining together alternately. (In reality, it isn't as simple as this - you may well just get them joining up randomly. But CIE want the two monomers to be shown in the repeat unit.) So if you combine vinyl acetate and ethene, you would get:
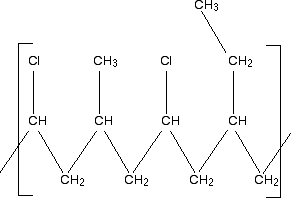
For condensation polymerisation This has all been covered in learning outcome 10.8(c). You may need to follow links from that page including the one to statement 10.7(k) and beyond. There are enough examples on these various pages to show how you get from the monomers of a condensation polymer to the structure of the polymer. Statement 10.8(g) This is about identifying the monomers which make up a given polymer. In principle, this shouldn't be any more difficult than reversing what you have done above. But, in fact, it can be quite scary, especially if you are given a really complicated looking structure. At the time of writing the scariest question involving this was June 2008 Paper 4 Question 7. I am going to look at similar examples to this, but leave you that question for practice. Example 1 Look at this repeating unit of an entirely imaginary polymer:
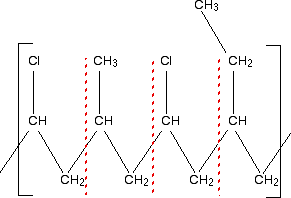
Notice to start with that the main chain consists only of carbon atoms. This must be an addition polymer. But notice also that there are several different side chains scattered along the main chain. This looks like a fairly complicated example of a copolymer. For an addition polymer, all you need to do is to split it up into two-carbon bits of chain working from the left-hand side.
That gives you the basic monomers. Don't forget to put the double bond back between the two carbon atoms. That means that the monomers of this polymer are:
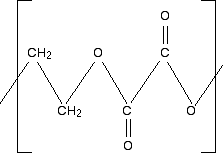
In an exam, you would obviously just write down the three monomers - not quoting the chloroethene twice. There is nothing difficult about this - just take the polymer to pieces in a systematic way. Example 2 What sort of polymer is this, and what are the monomers? Remember that this is the repeating unit. The chain will continue to the right and to the left in the same pattern.
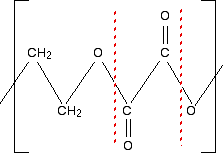
First of all, what sort of polymer is this? The presence of oxygens in the chain means that it is a condensation polymer, and you should also see the presence of ester links. So break the polymer up across the ester links into alcohol groups and either carboxylic acid groups or acyl chloride groups. That's what will have combined to make the polyester. If the question doesn't give you any clues about whether to write the monomer as a carboxylic acid or an acyl chloride, then you can choose whichever you like. The mark scheme will allow for either possibility. Break up the ester groups between the carbon-oxygen double bond and the single-bonded oxygen. That way you can most easily see where the alcohol groups are.
What are your two monomers? Remember that the left-hand carbon will be connected to an oxygen. (Think of drawing another repeating unit to the left of the one you are given.) So the alcohol is straightforward: HO-CH2CH2-OH. The acid will have an -OH group attached to each C=O bond: HOOC-COOH. Or, if you choose to draw an acyl chloride: ClOC-COCl. | |
|
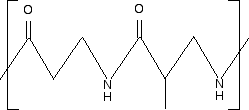
Note: You will find a question similar to this in the June 2008 Paper 4 that I mentioned above. The first structure in question 7 relates to a polyamide rather than a polyester, but everything else is the same. Except that it isn't the same, because the question is misleading. The structure between in brackets in the question is not the repeating unit. The left-hand CO and NH are not a part of the repeating unit - they are a part of the previous unit to the left. I would reasonably expect a student to be penalised for not drawing the repeating unit accurately, and I object strongly to this sloppiness from whoever wrote this question, and whoever checked it. Any student who took the diagram literally as the repeating unit couldn't get the answer given in the mark scheme.
I suppose I should complain to CIE about this, but long experience of Exam Boards tells me that it would be a waste of time! | |
|
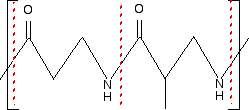
Example 3 I am going to make this one perhaps look more difficult by using skeletal formulae. What sort of polymer is this?
Remember that in a skeleton formula, at the end of each line or at a junction of lines, there is a carbon atom, and possibly some hydrogens, unless something else is drawn there. In this instance, there isn't a carbon at either of the junctions involving NH, but all the other junctions within the repeating unit do have a carbon atom. In a case like this, you can't assume that the extension bonds (the bonds passing through the brackets) necessarily have a carbon atom at the end. Imagine that you drew the same repeating unit to the left and to the right of the one shown. The bond coming out through the left-hand bracket would actually be attached to another NH. The one through the right-hand bracket would be attached to a carbon - with an oxygen double-bonded to it. So, again, what sort of polymer is this? The presence of the nitrogen in the main chain shows that it must be a condensation polymer. In fact it has amide links, and so is a polyamide. Now split the amide links in the same way we split the ester links in the previous example.
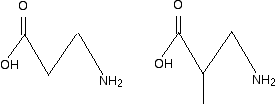
I have included the dotted red lines at both ends of the repeating unit to emphasis the fact that these are also amide links. Let's assume that the amide links in the polymer came from a -COOH group and an -NH2 group. (The polymer could equally well have come from a -COCl group and an -NH2 group.) You would have started from the following two monomers. In other words, this is another copolymer.
Important The essential thing when you look at examples like this is not to get panicked by them. I must admit that when I first looked at the June 2008 question that this section about statement 10.8(g) is based on, I just thought "Oh, no - I can't be bothered with this!" But if you take your time, and take the polymer chains to pieces as I have shown you, it really isn't that difficult. Obviously you have to be able to recognise ester and amide links in molecules, and know the sort of reactions that form them. Once you have got the basics sorted out, find as many examples as you can from past papers and practise them until they don't bother you any more. You will find that most examples aren't actually as complicated as those above, but with CIE you must always expect some complication! Good luck!
© Jim Clark 2011 (modified August 2013) |
|