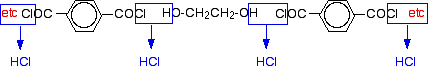|
Chemguide: Support for CIE A level Chemistry Learning outcome 10.8(c) This statement is about condensation polymerisation. Before you go on, you should find and read the statement in your copy of the syllabus. General Condensation polymerisation involves monomers joining together with the loss of a small molecule such as water or HCl. There are two general cases that you have come across where this happens. One involves the formation of ester linkages to give polyesters such as Terylene; the other involves the formation of amide linkages to give polyamides such as nylon. Proteins are also formed by condensation polymerisation of amino acids. Statement 10.8(c)(i) This is about the formation of polyesters like Terylene. Read the beginning of the page about polyesters. You do not need to know about their manufacture or hydrolysis. Work out the structure of the polyester by putting it together from the monomers as shown in the diagram with the water molecules being removed. You should also be aware that you could make polyesters using a molecule containing two -COCl (acyl chloride) groups as one of the monomers. The difference this time would be that you would lose HCl rather than water. For example, you could make Terylene by:
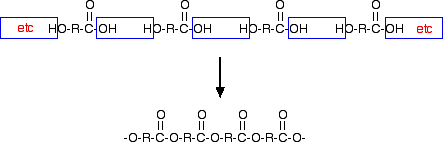
Yet another way of making a polyester (but not Terylene this time) would be to use a single monomer with a -COOH group at one end and an -OH group at the other. For example:
"R" could be any hydrocarbon group - a chain or a ring. There is no specific mention of this in either the syllabus or the teacher support material, but there was a question which expected you to recognise that a particular polymer structure came from a monomer like this. If you hadn't come across it in advance, you would have to be really good to spot this in an exam. Statement 10.8(c)(ii) This is about the formation of polyamides and polypeptides. You will probably remember that a peptide link is exactly the same as an amide link. Chemists call them amides; biologists and biochemists call them peptides. In both cases, you are forming polymers containing the link -CONH-. Start by looking at the page about learning outcome 10.7(l). Then look at the beginning of the page on protein structure. However, all of this protein stuff will be dealt with again in more detail in the Applications of Chemistry section 11.1. I would suggest that you leave anything beyond the second green box on that page until later.
© Jim Clark 2011 (modified August 2013) |