|
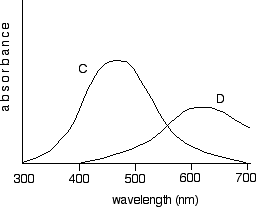
Chemguide: Support for CIE A level Chemistry Learning outcomes 9.5(k), 9.5(l) and 9.5(m) These statements are about the colours of transition metal complexes. Before you go on, you should find and read the statements in your copy of the syllabus. Basic material First read the page about the colours of complex metal ions. This will give you almost all of what you need for CIE purposes. About half-way down that page you will find a link to a further page which talks about the shapes of d orbitals and how this affects the way the energy levels of the d orbitals change when ligands are attached. It is essential that you follow this link because it covers what you need to know for statement 9.5(k). In case you have missed it, here is the link to the more about d orbitals page again. You should, however, read this at the point that it is placed on the main page. It won't make sense unless you have read the first part of that page first. Additional material for CIE Problems with statement 9.5(m) Refer back to the page about the colours of complex metal ions. About 3/4 of the way down the page you will find a list of ligands in order of how much splitting they cause. You will see that ammonia produces more splitting than water, and water produces more splitting than hydroxide ions, and hydroxide ions more than chloride ions. The syllabus statement mentions these complexes with copper(II) ions. The example which everyone concentrates on is the major colour difference between the pale blue (cyan) aqua complex, and the very dark blue complex with ammonia - and this is discussed on that page. The complex containing OH groups is more of a problem. This is the neutral complex that you get when you add hydroxide ions to [Cu(H2O)6]2+ ions. Its formula is Cu(H2O)4(OH)2. Most of the ligands here, of course, are still water, and there isn't much difference between the colour of the precipitate and that of the original hexaaquacopper(II) ion solution. I'm not sure exactly what CIE want here. Up to November 2012, nothing specific had been set on it. For teachers: There is also a major problem with a statement in the teacher support material. This says: "Students should be made aware that the more nucleophilic the ligand (i.e. more basic, as NH3 is compared to H2O), the larger the energy gap between the two sets of d orbitals is, and hence the higher is the frequency of absorption." OH- ions are much more powerful nucleophiles and are much more strongly basic than either water or ammonia. If this statement was true, then hydroxide ions would cause more splitting than either of the other two. That isn't the case. So that sentence in the support material must be wrong! The energy gap produced by a ligand isn't directly related to its abilities as a base or a nucleophile. CIE's questions about colour This is another good example of CIE's questions not following what it asks in either the syllabus or the teacher support material. The teacher support material follows the general line of my Chemguide page, and talks about the importance of the fact that the colour observed is the complement of the one absorbed. That means that you would need to learn the colour wheel shown on the Chemguide page. However, the questions asked so far on the whole couldn't be answered by working out the complementary colour, because you were given examples where the light absorbed covered a wide range of frequencies. These were given as an absorption spectrum - something that neither the syllabus nor the teacher support material mentions. One question gave you a graph like this for the absorption spectra of solutions of two transition metal complexes, C and D:
Absorbance is a measure of the amount of each wavelength being absorbed. The higher the point on the curve for a given wavelength, the more of that wavelength is being absorbed. What the question wanted to know was what colour the two solutions were likely to be. Your choices were yellow, red, green or blue. To help you, they included the following table:
Look at the graph labelled C. C is absorbing very strongly through most of the visible region of the spectrum. Although the spectrum peaks at somewhere between 450 - 500 nm, which is in the blue-green region, it is also absorbing a reasonable amount in the violet and yellow as well. Because it is absorbing light over such a wide range of colours, trying to think in terms of complementary colours doesn't really work. In this sort of case, you need to look at what wavelengths are actually getting through. In this case, there is least absorption in the red region of the visible spectrum. You are actually helped in this question by being given a small number of colours to choose between. One of those colours is red. And that's the right answer. Now try to work out what colour D is likely to be before you read on. D is absorbing strongly in the red and yellow regions, and least strongly in the violet and blue. Given the colour choices you have got, the only possible answer is blue. The other bit of this question asked which of C and D would have the higher energy gap between the two groups of d orbitals? This needs some careful thought. You have a choice between a complex which is absorbing most strongly in the red, or one absorbing most strongly in the blue-green area. The table tells you that the energy of a photon of light is higher for blue or green light than for red. The energy absorbed is due to the energy gap between the two groups of d orbitals. Energy is taken out of the light to promote an electron from the lower group to a space in the higher one. The energy needed to absorb blue or green light is higher than the energy needed to absorb red light. So the gap must be bigger in the one which absorbs blue or green light - in other words, in C. The relationships between wavelengths, frequency and energy You were helped in this particular question by being given the trend in energy over a set of wavelengths. That won't necessarily be the case. You will probably be given the information you need, but you can't be sure of that.
© Jim Clark 2011 (last modified August 2013) |
|||||||||||||||||||