|
Chemguide: Support for CIE A level Chemistry Learning outcomes 9.5(h), 9.5(i) and 9.6(j) These statements are about transition metal complexes. Before you go on, you should find and read the statements in your copy of the syllabus. I am taking these three statements together because they are all part of the same topic. In particular, it is impossible to do 9.5(h) before you have done 9.5(i). Introduction There is far more about complex ions on Chemguide than you will need for CIE purposes. So I am going to ask you to read some Chemguide material as background, and then I will pull all the specific information that you need together on this page. First read the page introducing complex metal ions. Don't try to remember all of this - there is more there than you really need. What you need to do is to understand what is going on. For CIE purposes, the most important things to know in this introductory bit is that:
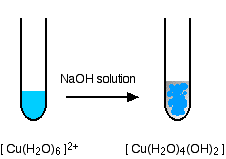
Complexes don't have to involve a transition metal. You will find an example of an aluminium complex ion on that page. It is more common with transition metals, though. Now read the page about the shapes of complex ions. You don't need the later part about stereoisomerism, although it might pay you to read the small bit about cis-trans isomerism in cisplatin. The page gives you examples of octahedral, tetrahedral and square planar complexes, but not a linear one - which the syllabus also wants. The simplest of these is the diamminesilver(I) ion, Ag(NH3)2+. The silver ion is in the middle with the two ammonia molecules joined on at 180°. They are joined using lone pairs on the two nitrogen atoms bonding to empty orbitals on the silver ion - dative covalent bonding again. Then read the page about naming complex metal ions. The names look scary, but are actually less complicated than the names of organic compounds. Again, make sure that you understand this page, so that if you come across a name, you aren't frightened by it. Ligand exchange reactions Look at this page about ligand exchange. Again, you don't need to remember all of this, but you should understand what ligand exchange means. Concentrate particularly on the examples involving copper, because that is what you are most likely to be asked about (see below). But read the rest of the page as well. Ignore any link which offers to explain anything in more detail! Complex ions in copper chemistry These are mentioned in both of the syllabus statements we are looking at, and come up frequently in exams. The reaction of hexaaquacopper(II) ions with hydroxide ions Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. The hydroxide ions are acting as a base, and the hexaaqua ion is acting as an acid. That is typical of ions like this. Once a hydrogen ion has been removed from two of the water molecules, you are left with a complex with no charge - a neutral complex. This is insoluble in water and a precipitate is formed.
| |
|
Note: The colour coding is to show that this isn't a ligand exchange reaction. The oxygens which were originally attached to the copper are still attached in the neutral complex. | |
|
This neutral complex is what is normally known as copper(II) hydroxide. We often write this just as Cu(OH)2, ignoring the waters present. In the test-tube, the colour change is:
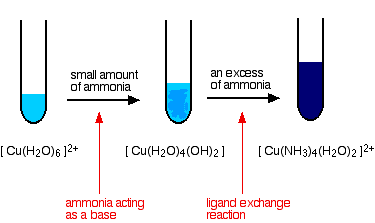
Reactions of hexaaquacopper(II) ions with ammonia solution The ammonia acts as both a base and a ligand. With a small amount of ammonia, hydrogen ions are pulled off the hexaaqua ion exactly as in the hydroxide ion case to give the same neutral complex.
That precipitate dissolves if you add an excess of ammonia. The ammonia replaces water as a ligand to give tetraamminediaquacopper(II) ions. Notice that only 4 of the 6 water molecules are replaced.
| |
|
Note: In fact this reaction is better looked at as the replacement of 4 of the water molecules in the hexaaquacopper(II) ion, [Cu(H2O)6]2+. However, here I am using the equation for this reaction that is given by the CIE teacher support material and also in the Chemistry Coursebook. In fact, this is a summary of a complicated series of equilibria. If your chemistry is good, and you are interested, you will find the explanation in full on the page about the reactions between hexaaqua ions and ammonia solution. You do not need to know this for exam purposes, though. Use the BACK button on your browser to return to this page. | |
|
The colour changes are:
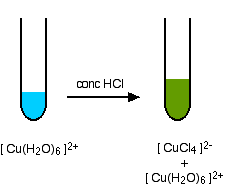
It is possible to reverse all of this by adding a dilute acid such as sulphuric acid to the deep blue solution. The acid first reacts with the ammonia, reversing the last equation. You go back to the neutral complex. The blue precipitate of copper(II) hydroxide is formed again. Adding more acid puts the hydrogen ions back on the two OH groups again, and so you get back to the pale blue soluble hexaaqua complex. A ligand exchange reaction involving chloride ions If you add concentrated hydrochloric acid to a solution containing hexaaquacopper(II) ions, the six water molecules are replaced by four chloride ions.
The reaction taking place is reversible.
Because the reaction is reversible, you get a mixture of colours due to both of the complex ions. You may find the colour of the tetrachlorocuprate(II) ion variously described as olive-green or yellow. If you add water to the green solution, it returns to the blue colour. The equilibrium is easily reversed by adding more water. | |
|
Note: If you dissolve copper(II) oxide in concentrated hydrochloric acid (to make copper(II) chloride which will then go on to form the tetrachlorocuprate(II) ion), you get a dark yellowish brown solution. This probably better reflects the colour of the ion. CIE described it as yellow-green in the only question I could find up to November 2010. | |
|
The question that CIE set involving this complex asked about the effect of adding concentrated ammonium chloride solution to copper(II) sulphate solution. You were told that this produced yellow-green crystals with an empirical formula of CuN2H8Cl4, and were asked to explain what was happening. If you hadn't come across this reaction before, you are likely to get this wrong under the pressure of exam conditions. The crystals formed are actually ammonium tetrachlorocuprate(II), (NH4)2CuCl4. This is a ligand exchange involving the chloride ions from the concentrated ammonium chloride solution. The trap to fall into is to think that it must be a ligand exchange involving ammonia - and that's the trap which the Examiner's Report showed that many people did in fact fall into. It looks to me as if the question was deliberately set to persuade people to fall into this trap. If you stopped to think about it, it can't be an exchange involving ammonia for two reasons. The colour is wrong (the ammino complex is very dark blue), and you have ammonium ions, not ammonia present. Ammonium ions don't have a lone pair of electrons, and so can't act as ligands. It isn't always easy to think that clearly under exam conditions, so it helps if you have come across this reaction in advance. Well . . . now you have! Some other bits and pieces of copper chemistry I include this here, not because it is on the syllabus at this point (it isn't!), but because it has come up in questions about the copper chemistry we have talked about above. It involves the effect of heat on copper(II) carbonate and copper(II) nitrate. You will have come across the effect of heat on nitrates and carbonates as a part of statement 9.2(g), but this relates to Group 2, and you may not have made the link that it also applies to transition metal carbonates and nitrates. The effect of heat on copper(II) carbonate Copper(II) carbonate is a green powder which turns black when heated, and gives off a colourless gas which turns lime water milky (carbon dioxide). The black residue is copper(II) oxide.
The effect of heat on copper(II) nitrate Hydrated copper(II) nitrate is blue crystals, but the question which asked about this talked about the effect of heat on anhydrous copper(II) nitrate which is also apparently blue. You weren't asked for this colour, and the question didn't give it, either. Either form turns black when heated, and gives off the brown gas, nitrogen dioxide, and oxygen. Oxygen, of course, relights a glowing splint. The black residue is again copper(II) oxide.
© Jim Clark 2011 (last modified August 2013) |
|