|
Chemguide: Support for CIE A level Chemistry Learning outcome 9.5(f) This statement looks at various typical redox reactions involving transition metal compounds. Before you go on, you should find and read the statement in your copy of the syllabus. Writing equations for redox reactions This statement will expect you to work out the equations for redox reactions using half-equations. If you aren't confident about this, then you must read the page about writing ionic equations for redox reactions before you go on. For CIE purposes, you probably won't have to work out the half-equations for yourself. For inorganic reactions, you will probably either be given the half-equation, or you can find it from the Data Booklet which you will have in the exam. You will find a copy of this booklet towards the end of the syllabus. For organic reactions, you probably won't be asked to write these ionic equations anyway. The teacher support material says that oxidation of, for example, alcohols can be written in terms of [O] instead of a proper equation. Example 1 One of the examples that you might have just read about on the page about writing ionic equations was the reaction between acidified potassium manganate(VII) and hydrogen peroxide. Oxygen is produced, and the manganate(VII) ions are reduced to manganese(II) ions. In the example on that page, you had to work out the half-equations for yourself. If you have the Data Booklet, how do you go about finding them? First, find the copy of the Data Booklet towards the end of the syllabus, and find the table headed "Standard electrode potential and redox potentials". There are two versions of this table. You want the first one - in alphabetical order. Then you need to think about the redox reaction in two halves. You need to find an equation which involves MnO4- ions and Mn2+ ions (and hydrogen ions as well, because it is done under acidic conditions). All the reactions in this table are shown as equilibria. That means that you could make them move in either direction. So it doesn't matter which side of the equation the MnO4- and Mn2+ appear on - as long as they are on opposite sides. Look through the table (bearing in mind that it is in alphabetical order), and you should find:
That is actually the change you want to happen, so you can replace the reversible sign by a one-way arrow.
Now do the same thing for the hydrogen peroxide reaction. You need an equation with hydrogen peroxide on one side and oxygen on the other. You should find:
That's the reverse of what you want to happen, so turn it around and replace the reversible sign with a one-way arrow.
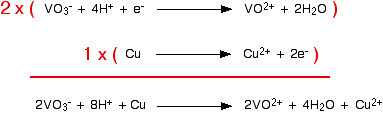
Now you can combine these equations together as you were shown on the page about working out ionic equations for redox reactions. This is good practice. Do it now and then look at that page again to see if you got it right. If you didn't, spend some more time on that page before you go any further. Example 2 This comes from a past paper and was worth 2 marks. You were told that copper reacts with sodium vanadate(V) solution, NaVO3, to give a solution containing VO2+ ions and Cu2+ ions, and had to work out the equation. Because of the formula of the sodium vanadate(V), the vanadate(V) ions must carry a charge of -1. So the first thing you have to do is to find a half-equation for the change from VO3- to VO2+. That's not difficult. You should find:
That is the direction you want it to go in, so you can change it to:
You could reasonably make up the copper half-equation for yourself.
Or you could find it in the table. You would have to remember to reverse the direction, though. Now all you have to do is to add them together, remembering that you need to make sure that the number of electrons being given and received by the two equations is the same. The vanadate(V) reaction would have to happen twice to make this work.
The Fe3+ / Fe2+ system You are probably most likely to come up against this during a titration with either potassium manganate(VII) solution or potassium dichromate(VI) solution. Those are both covered below. There are lots of things which will oxidise Fe2+ to Fe3+, and several reducing agents which will reduce Fe3+ to Fe2+. We will look at examples of these in statement 9.5(g) which talks about E° values. The MnO4- / Mn2+ system Manganate(VII) ions (permanganate ions) are very powerful oxidising agents, and are used in both organic and inorganic reactions. You will find this discussed on the page about manganese chemistry. You can start reading from the title "Some potassium manganate(VII) chemistry". Ignore the earlier stuff. Titrations In the introduction to the titrations, it would be good for you to practice working out the equations for all the reactions mentioned, starting from the various half-equations. How do you know if you are right when you have done it? Check that the numbers of atoms and charges on both sides balance. As a hint, in all but the iron case, you will have to think about the transfer of 10 electrons. And don't forget to simplify the equations by sorting out things like hydrogen ions and water which may appear on both sides. This is all done for you for hydrogen peroxide on the page about writing ionic equations for redox reactions. The teacher support material specifically mentions the use of potassium manganate(VII) titrations to estimate amounts of iron(II) ions. So make sure that you can work out that reaction's equation. It also mentions the use of these titrations to estimate the amount of iron itself. Suppose you had some impure iron of a certain mass. If you dissolved this carefully in some dilute sulphuric acid, the iron will dissolve to give iron(II) ions in solution. You might then make this up to, say, 250 cm3 with pure water. If you then took a 25 cm3 sample of this and acidified it with more sulphuric acid, you could titrate it with standard potassium manganate(VII) solution, and use the results to find out how many moles of iron(II) ions you had in the original solution. That will be the same as the number of moles of iron in the impure iron. So you can work out the mass of the actual iron in your impure iron, and therefore the percentage purity of the iron. If you have access to a copy, you will find a worked example of this on page 70 of my calculations book, or a slightly simpler example on page 366 of the Chemistry Coursebook. Organic Potassium manganate(VII) isn't a good oxidising agent to use in organic chemistry, because it is powerful enough to break carbon-carbon bonds. Both of the examples on the manganese chemistry page are mentioned by the syllabus, though. In addition to this, you should read the page about statement 10.2(d) which also deals with the effect of hot concentrated potassium manganate(VII) on alkenes. Look particularly at the part about 10.2(d)(iii). You must expect to get asked questions about this. The Cr2O72- / Cr3+ system You will find much more than you will need on the page about chromium chemistry. You can ignore everything on the first half of the page until you get to the heading "The reduction of dichromate(VI) ions with zinc and an acid". From then on, you will need parts of the page - but by no means all of it. Use the guidance below as you go along. The Cr2O72- / Cr3+ half-equation You can find this from the Data Booklet which you will have in the exam. There is a copy towards the end of the syllabus. It is difficult to get confused about this one! It is:
Whenever you come across an equation involving the change from dichromate(VI) ions to chromium(III) ions on the page, make sure that you can work it out. The reduction of Cr2O72- with zinc and acid It is possible that this could come up, although you wouldn't be likely to need the final step of the reduction to blue chromium(II) ions. These aren't mentioned by the syllabus or the teacher support material. Organic chemistry You won't need the proper equations - the ones with [O] will do for CIE. You can ignore the bit about making chrome alum crystals. Titrations You could be asked about potassium dichromate(VI) titrations, especially as a calculation question, although there is no reference to the particular indicator used anywhere in the syllabus or teacher support material, and so you shouldn't be asked for experimental detail. No such question had been asked up to November 2012. You can ignore the test for chromate(VI) ions at the bottom of the page.
© Jim Clark 2011 (modified August 2013) |