|
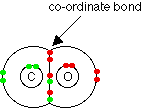
Chemguide: Support for CIE A level Chemistry Learning outcome 9.3(d) This statement covers a lot of chemistry of the oxides of Group 4 elements. Before you go on, you should find and read the statement in your copy of the syllabus. In all honesty, this is boring stuff - but it is an area where CIE tend to ask questions. However boring it is, you must take the trouble to learn as much of it as you can. You should check as many past papers as you can so that you can see which are the things most likely to come up, and how much you need to learn as opposed to working it out if you have to. The oxides The elements form two ranges of oxides - monoxides and dioxides. That is because the elements have two possible oxidation states, +2 and +4. Monoxides include carbon monoxide, CO, tin(II) oxide, SnO, and lead(II) oxide, PbO. Dioxides include CO2, SiO2, SnO2 and PbO2. The two non-metal oxides would normally be called carbon dioxide and silicon dioxide. The metal oxides would normally be called tin(IV) oxide and lead(IV) oxide. But you may find the names used fairly interchangeably, so that lead(IV) oxide is often called lead dioxide, for example. Lead also has another oxide with the formula Pb3O4, called trilead tetroxide. This is a bright red solid known popularly as "red lead". Pb3O4 behaves chemically as if it were a mixture of PbO and PbO2 in the ratio 2PbO.PbO2. You will come across this again later on this page. Bonding in the oxides In the non-metallic elements from carbon to germanium, the oxides are covalent. For CIE purposes, you can think of the metal oxides (of tin and lead) as being ionic. The CIE teacher support material says that SnO2 and PbO2 are most easily described as ionic, but with a degree of covalency - and so that is what you need to learn. It is worth looking separately at a few of the oxides in more detail. The oxides of carbon The fact that the two oxides of carbon are gases must mean that they are simple molecular substances. Carbon monoxide can be thought of as having two ordinary covalent bonds between the carbon and the oxygen plus a co-ordinate bond using a lone pair on the oxygen atom.
In carbon dioxide, carbon forms simple molecules with oxygen because of the possibility of carbon-oxygen double bonds.
None of the other elements in Group 4 form double bonds with oxygen, and so that forces completely different structures on them. Silicon and germanium dioxides Neither of these elements form double bonds with oxygen. | |
|
Mainly for interest: Silicon atoms are bigger than carbon atoms. That means that silicon-oxygen bonds will be longer than carbon-oxygen bonds. To make a double bond, you have to have sideways overlap between p orbitals on adjacent atoms. With the longer silicon-oxygen bonds, the p orbitals on the silicon and the oxygen aren't quite close enough together to allow enough sideways overlap to give a stable pi bond. So, silicon bonds with oxygen in such a way that only single bonds are formed. Similarly with germanium. | |
|
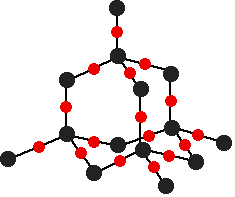
There are various different structures for silicon dioxide. The easiest to remember and draw is:
This is based on a diamond structure with each of the silicon atoms being bridged to its other four neighbours via an oxygen atom. This means that silicon dioxide is a giant covalent structure. The strong bonds in three dimensions make it a hard, high melting point solid. Exactly the same is true of germanium dioxide. The tin and lead oxides If you think of these as ionic, then SnO and PbO will have giant ionic lattices consisting of Sn2+ or Pb2+ ions and oxide ions. SnO2 and PbO2 will have giant ionic lattices consisting of Sn4+ or Pb4+ ions and oxide ions. The acid-base behaviour of the oxides Before we get bogged down in detail, here is a summary of the important points.
The oxides of carbon Carbon monoxide is only slightly soluble in water and doesn't react with it. It is normally counted as a neutral oxide. CIE describe it as neutral in their teacher support material - and so that is what you must learn. | |
|
Note: Carbon monoxide does in fact react with hot concentrated sodium hydroxide solution to produce sodium methanoate. The fact that the carbon monoxide reacts with the basic hydroxide ion shows that it must be acidic. | |
|
Carbon dioxide is weakly acidic. With water Carbon dioxide reacts with water to a slight extent to produce hydrogen ions (strictly, hydroxonium ions) and hydrogencarbonate ions. Overall, this reaction is:
The solution of carbon dioxide in water is sometimes known as carbonic acid, but in fact only about 0.1% of the carbon dioxide has actually reacted. The position of equilibrium is well to the left-hand side. With bases Carbon dioxide reacts with sodium hydroxide solution in the cold to give either sodium carbonate or sodium hydrogencarbonate solution - depending on the reacting proportions.
Silicon dioxide Silicon dioxide doesn't react with water, because of the difficulty of breaking up the giant covalent structure. Silicon dioxide does react with sodium hydroxide solution, but only if it is hot and concentrated. Sodium silicate solution is formed.
Silicon dioxide is weakly acidic. Germanium, tin and lead oxides The monoxides All of these oxides are amphoteric - they show both basic and acidic properties. The basic nature of the oxides is shown by their reaction with acids to form salts. For example, they all react with hydrochloric acid. This can be summarised as:
For example, with tin(II) oxide, you get tin(II) chloride and water.
The acidic nature of the oxides is shown by their reaction with bases like sodium hydroxide solution. In general terms:
For example, with tin(II) oxide, you get sodium stannate(II) solution and water. The stannate name is given to ions where tin is a part of the negative ion. The (II), of course, shows the tin in the +2 oxidation state.
| |
|
Note: There are various versions of the formulae for compounds like sodium stannate(II). This is the simplest one, and is acceptable to CIE. | |
|
The dioxides These dioxides are again amphoteric - showing both basic and acidic properties. The basic nature of the dioxides is shown by their reaction with concentrated hydrochloric acid to give compounds of the type XCl4:
| |
|
Note: The XCl4 actually goes on to react with excess chloride ions in the hydrochloric acid to give complexes such as XCl62-, but this isn't required by CIE. | |
|
In the case of lead(IV) oxide, the reaction has to be done with ice-cold hydrochloric acid. If the reaction is done any warmer, the lead(IV) chloride decomposes to give lead(II) chloride and chlorine gas. This is an effect of the preferred oxidation state of lead being +2 rather than +4. The reaction with hydrochloric acid isn't typical of lead(IV) oxide, which doesn't react with most acids. The acidic nature of the dioxides is shown by their reaction with hot concentrated sodium hydroxide solution or molten sodium hydroxide. In general terms:
For example, with tin(IV) oxide, you get sodium stannate(IV) and water.
| |
|
Note: As in the case of the similar compound formed with tin(II) oxide, there are various formulae for the product in this reaction. This is the simplest, and is acceptable to CIE. | |
|
The special case of Pb3O4 You may remember that Pb3O4 can be thought of chemically as a mixture of 2PbO.PbO2. If you react this with an acid, then the two oxides which can be thought of as making up the Pb3O4 behave independently. For example, the PbO will react with nitric acid to give lead(II) nitrate solution and water:
However, the lead(IV) oxide doesn't react with nitric acid. Thinking of Pb3O4 as 2PbO.PbO2, the PbO would react with the nitric acid, and the PbO2 would be left over.
| |
|
Care! This equation came up in a CIE question. The examiner's report said that many candidates forgot to multiply the lead(II) oxide part of the equation by two. That would be an annoying mistake to make, because you have already done the hard part by working out or remembering that it can be thought of as 2PbO.PbO2. | |
|
The thermal stability of the oxides This is about the way the oxides are affected by heating. The monoxides CO, SiO and GeO Apart from carbon monoxide, these oxides are unimportant for A level chemistry purposes. They all disproportionate on heating - that is, one molecule undergoes both oxidation and reduction. For example, in the case of SiO, one molecule is reduced to silicon while the other is oxidised to silicon dioxide. They all undergo the change:
In the case of carbon monoxide, a catalyst is needed even at relatively high temperatures. These changes reflect the fact that the +4 oxidation state (in the dioxides) is more stable than the +2 state at the top of Group 4. SnO and PbO These are stable to heat. The dioxides The dioxides are stable to heat except for PbO2. This decomposes on heating to give lead(II) oxide and oxygen.
This reflects the greater stability of the +2 oxidation state at the bottom of Group 4. Pb3O4 (thought of as 2PbO.PbO2) will, of course, also give off oxygen on heating to give simple PbO.
© Jim Clark 2011 (modified August 2013) |
|