|
Chemguide: Support for CIE A level Chemistry Learning outcome 9.3(a) This statement is about the bonding and structures of the Group 4 elements, and how this affects their melting points and electrical conductivity. Before you go on, you should find and read the statement in your copy of the syllabus. If you have been working through the Chemguide CIE pages, you will know that, as far as possible, I have referred you to existing Chemguide pages. I have decided not to do this for Group 4. The Chemguide Group 4 pages contain a lot more information than you will need for CIE, and there is a risk that you will get confused about what you need to know and what you can ignore. So all the pages in this section 9.3 are specifically produced for the CIE syllabus, although you will find a lot simply copied from the existing Chemguide pages. Structure and bonding Structures of the elements There is a trend from non-metal to metal as you go down the Group which is clearly seen in the structures of the elements. Carbon at the top of the Group has giant covalent structures in its two most familiar allotropes - diamond and graphite. | |
|
Allotropes: Two or more forms of the same element in the same physical state. The structures of diamond and graphite are explored in more detail on a page about giant covalent structures on Chemguide. It would probably be worth your while to read this page before you go any further. Use the BACK button on your browser to return quickly to this page. | |
|
Diamond has a three-dimensional structure of carbon atoms each joined covalently to 4 other atoms. The diagram shows a small part of that structure.
Exactly this same structure is found in silicon and germanium and in one of the allotropes of tin - "grey tin" or "alpha-tin". The common allotrope of tin ("white tin" or "beta-tin") is metallic and has its atoms held together by metallic bonds. The structure is a distorted close-packed arrangement. In close-packing, each atom is surrounded by 12 near-neighbours. By the time you get to lead, the atoms are arranged in a straightforward 12-co-ordinated metallic structure. | |
|
Note: If you aren't sure about metallic bonding or metallic structures, you should follow these links before you go any further. The first link will actually lead you to the second one if you want to explore both of these topics. Use the BACK button on your browser to return to this page. | |
|
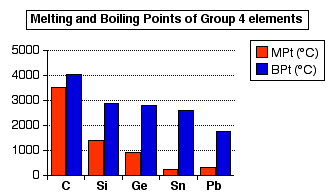
So the bonding in the elements at the top of the Group is giant covalent. At tin, there are two forms, one of which is giant covalent, and one of which is metallic. At lead, the bonding is entirely metallic. Physical properties of the elements CIE expect you to be able to describe and explain the variation in melting point and electrical conductivity in terms of the changes in the structure and bonding of the elements as you go down Group 4. Trends in melting point The following bar chart shows the trends in both melting and boiling points. Although they aren't mentioned by CIE, boiling points actually give a better indication of interatomic forces than melting points do. The bonds aren't fully broken until you boil the substance.
You will see that the trend is for melting and boiling points to fall as you go down the group. | |
|
Note: The low value for tin's melting point compared with lead is presumably due to tin forming a distorted 12-co-ordinated structure rather than a pure one. The tin values in the chart refer to metallic white tin. | |
|
Comparing carbon, silicon and germanium (all of which have giant covalent structures), the melting points fall because the atoms are getting bigger. That means that the bonding pairs of electrons are further from the nuclei, and so the bonds are weaker. It would be dangerous to make a simple comparison between these first three elements and tin and lead, because the bonding is different - metallic rather than covalent. Obviously, though, these metallic bonds are weaker than the covalent bonds in the first three elements because the melting and boiling points are lower. The boiling pint of lead is lower than that of tin as you would expect. The atoms are bigger, and a longer bond is a weaker bond. Electrical conductivity You have to look at the elements on a case-by-case basis. Carbon Carbon as diamond doesn't conduct electricity. In diamond the electrons are all tightly bound and not free to move. However, carbon as graphite does conduct. The reason (not wanted by CIE) is that in the graphite structure each atom donates one electron to a delocalised system of electrons which takes in the whole of its layer (rather like an extended benzene). These electrons are free to move around, and so graphite conducts electricity - but this is a special case. Silicon and germanium Silicon and germanium are semiconductors. The theory of semiconductors is beyond A level chemistry, but if you are interested in a brief introduction, then you could read the big green box about half-way down the page about the trend from non-metal to metal in Group 4. For CIE A level chemistry purposes, you can pick out whether a substance is a normal conductor or a semiconductor by the effect of heat on the resistance of the material. In metals, the conductivity falls if you increase the temperature. In semiconductors, the conductivity increases at higher temperatures. Tin and lead White tin (normal metallic tin) and lead are good metallic conductors of electricity. The only clear-cut cases here are diamond and the two metals. In diamond, the covalent bonding stops the electrons moving. In tin and lead, you have delocalised electrons. For silicon and germanium, you can say that they aren't normal conductors because they don't have any delocalised electrons, due to the covalent bonding. But without going into the theory of semiconductors, for A level chemistry, it is impossible to explain why they act as semiconductors.
© Jim Clark 2011 (modified August 2013) |
|