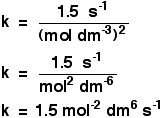|
Chemguide: Support for CIE A level Chemistry Learning outcomes 8(g) and 8(i) These statements are about rate equations and orders of reaction. I am combining them because 8(i) comes naturally out of the work on 8(g). It also includes some terms from statement 8(a). Before you go on, you should find and read the statements in your copy of the syllabus. This is going to be a very long page! Important background First read the page orders of reaction and rate equations. Ignore the link near the bottom of the page to a further look at rate constants - this isn't required by CIE. Statement 8(a) asks you to explain and use the term order of reaction. Using it isn't very difficult; explaining it is quite difficult to do quickly in terms of a short definition on a structured exam paper. I would be fairly surprised if you were asked to do this, but if you were, there isn't a simple definition in words which allows you to easily distinguish between the orders with respect to individual substances and the overall order of the reaction. Instead, I would write a general rate equation as shown on the page you have just read, and then just say that the terms "a" and "b" are known as the orders of reaction with respect to A and B, and that the overall order of the reaction equals a+b. In other words, you explain it mainly in symbols rather than in words. I would do something similar for the term rate constant as well - write a general rate equation, and point out that the symbol k is called the rate constant. Statements 8(g)(i) and 8(i) These statements are about finding orders of reaction and rate constants from concentration-time graphs, initial rate experiments and half-life methods. There is no Chemguide page about this because it is covered in detail in my chemistry calculations book on pages 127 to 136, and I can't repeat it here. All I can do is to give you a brief outline of how to do it. From concentration-time graphs Finding the order of reaction from a concentration-time graph can be a seriously tedious thing to do. It isn't clear from the syllabus what depth is required, and so I contacted CIE about it. All they want is for you to be able to recognise the graph for a first order reaction because it has a constant half-life. Obviously, you can't understand this until you know about half-lives which first appear in statement 8(h). I will leave talking about this until then. From initial rate experiments When you do a reaction, it tends to start off quickly and then slow down as the reagents get used up. If you measure the rate at the very beginning of the reaction, you actually know exactly what the concentrations of all the reagents are at that time, because nothing has been used up yet. If you then change the concentrations you are using, and repeat the experiment, you can relate any change in rate to the what you have done to the concentrations. This is easier to see with an example. Example 1 This is the sort of data you might be given. Remember that [A] means the concentration of A in mol dm-3.
In simple cases like this, you can just look at the numbers. When you double the concentration of A between experiments 1 and 2 (keeping [B] constant), you double the rate. The rate is proportional to [A], and so the reaction is first order with respect to A. Between experiments 2 and 3, you keep the concentration of A constant, but double [B]. The rate goes up 4 times. So the rate is proportional to the square of the concentration of B, and the reaction is second order with respect to B. That means that the rate equation is: Rate = k[A][B]2 You can now go on to use this to find a value for the rate constant, k. All you need to do is to substitute values for the rate and the concentrations into this equation and solve it for k. Which experimental values do you use? It doesn't matter. Whichever you use will give the same value for k. It makes sense to use the easiest looking values, such as experiment 1, but it really doesn't matter. Prove it to yourself by doing the calculation three times if you can be bothered. Taking values from experiment 1: 0.0015 = k x 0.10 x 0.102 Rearranging this to find k gives a value for k of 1.5 But you can't leave it there. It has to have some units. Unfortunately, the units vary depending on the overall order of the reaction, and you have to work them out each time. There are two ways you can do this: When you substitute the numbers into the equation, include all of their units as well. So in this instance, the expression for k would be:
Then, as well as working out the numbers, you also cancel and rearrange the units. That gives:
Personally, I find it slightly less confusing (and I am also slightly less likely to make mistakes) if I process the numbers and the units separately. So I would do the calculation just with the numbers in the expression, and then repeat it writing the units instead of the numbers. The first line of working out the units would be:
Then you just cancel and rearrange the units as above. In an exam, you wouldn't have to show how you worked out the units - you just have to get them right! It will be worth a mark, and you can't afford to waste one of those in a CIE exam. | |||||||||||||||||
|
Note: I have explained this in much more detail on pages 129 - 130 of my chemistry calculations book, and with detailed working on later examples as well. | |||||||||||||||||
|
Example 2 CIE, being CIE, are much more likely to confuse things, of course! This example is similar to one set by CIE, and you wouldn't want to meet this for the first time in an exam.
You are forced to start with experiments 1 and 2, because those are the only ones where one of the concentrations is constant. This time the relationship isn't as simple as a doubling. Find out how many times the concentration of B has increased by dividing 0.24 by 0.16. It has gone up 1.5 times. Now do the same thing for the rate. That has gone up 1.5 times as well, and so the reaction is first order with respect to B. That's not too difficult. The more confusing thing is working out the order with respect to A, because the concentration of B is never held constant. Choose a pair of reactions where the concentration of B has changed in some simple way (if possible). It doesn't really matter, but you might as well make life as easy for yourself as you can. In this example, it would make more sense to choose experiments 1 and 3 where its concentration has doubled. (If you chose 2 and 3, its concentration has gone up 1.333333(etc) times. That feels more awkward!) If the concentration of A had stayed the same between experiments 1 and 3, the rate would have doubled because of the doubling of [B]. In other words it would have increased to 0.0028. But, in fact, it has gone up to 0.0035. This additional increase is because of the increase in the concentration of A. How many times has the rate increased due to A? Divide 0.0035 by 0.0028. (Remember that 0.0028 is what the rate would have been if you just doubled the concentration of B.) It has gone up 1.25 times due to the change in concentration of the A. If you divide 0.15 by 0.12 to find out how many times the concentration of A has gone up between experiment 1 and 3, you will find that is also 1.25 times. So it is first order with respect to A as well. That means that the rate equation is:
By substituting numbers from any one experiment into this equation, you can go on to find the value of the rate constant (together with its units.) If you work this out, you will find that k = 0.073 mol-1 dm3 s-1. The answer is given to 2 significant figures because the numbers you are using to work it out are all to that degree of accuracy. Work this out for yourself (including the units) to be sure that you can do it. The best way of learning to do these questions (as with all numerical problems) is to practise doing lots and lots of them. From half-lives You obviously can't do this until you know about half-lives. This is covered in statement 8(h), and you will find examples there of working out rate constants from half-lives. Statement 8(g)(ii) This is about recognising either a zero order or a first order reaction from a graph showing how the concentration of one of the reactants changes with time. It is impossible to make sense of this without first doing some work on later statements in Section 8. I will discuss this as a part of statement 8(h). Statements 8(g)(iii) and (iv) These statements are about the relationship between orders of reaction and reaction mechanisms. Start by reading the page orders of reaction and mechanisms. You don't specifically need to know about molecularity. Just read that bit of the page quickly so that you can refer back to it in case you come across another source that uses the term. In the section titled "More about reaction mechanisms and orders of reaction", by sure that you understand how to do problems where the slow step is the first step. I am not entirely clear what CIE expect in reactions where the slow step isn't the first step. The new CIE A level book (published January 2011) passes over this briefly in a very few lines, but with no detailed discussion. It makes no reference to equilibria. And there is no indication from the syllabus or teacher support material that this is wanted. The first question CIE set about this since the beginning of the current set of syllabuses in 2007 was November 2008, Paper 4, Question 2(b), and was seriously difficult. More recent questions have been more straightforward. The following is essentially a criticism of the November 2008 question. Unless you are reasonably confident, you could perhaps skip this, and go on to the section about 8(g)(v) below it (but read the three paragraphs just above that section before you go on). The question is about the reaction:
It doesn't matter whether you have come across this before or not - it could just as well have been in terms of A, B, C, etc. It was suggested in the question that the mechanism was in three steps:
You were then asked to work out what the orders would be with respect to the three substances on the left-hand side of the original equation. Unfortunately, you had to do this three times over, depending on which of the three steps was the slow one. Each time earned you one mark. If the first step is the slow one, there is no difficulty. Anything that happens in the later fast steps is irrelevant. The first step has a molecule of hydrogen peroxide reacting with an iodide ion - one of each. The reaction will be first order with respect to both of these, but zero order with respect to the hydrogen ions which don't appear until later, in fast steps. I would like to think that most people could get the mark for doing this. After that it gets more difficult. The Examiner's Report for this part of the question said "Candidates rarely scored full marks here. Most candidates failed to appreciate that all reactions, other than the slow step, were to be considered as equilibria." I don't understand this! First of all, it isn't surprising that most candidates didn't think of any of the stages as reversible when they were all written with one-way arrows. There is no suggestion in the syllabus or the teacher support material that students should have to relate this to equilibria. Secondly, if they really wanted you to work it out properly, it is quite tricky, and would take some time. Just awarding 1 mark for all this work can't be enough. The problem of thinking of this in terms of equilibria as I have done on the Chemguide page is made awkward by the presence of the water on the right-hand side of the first step. The reaction is done in solution in water. That means that any additional water formed during the reaction is going to make virtually no difference to the concentration of the water. You have to realise that the concentration of the water will be virtually constant because it is there in huge excess, because it is the solvent for the reaction. If you don't realise that, however long you spend on the problem, you can't get the answer in the mark scheme. I don't think many students who tried to do this properly would actually realise that! The only way you can get the answers in the mark scheme is to use a faulty approach. For example, if the second stage was the slow one, the concentration of IO- would depend on the concentrations of hydrogen peroxide and iodide ions. So you might make the assumption that the rate of the second step would be proportional to the concentrations of hydrogen peroxide and iodide ions (from the first step) and hydrogen ions (from the second step) - first order with respect to all of them. Another possible approach (equally faulty) is perhaps suggested by a comment in the teacher support material: "In multi-step reactions, the concentrations of any species involved after the rate determining step do not appear in the rate equation."
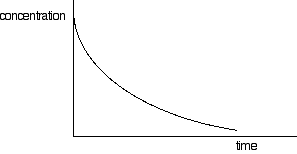
By the second step (if that is the slow one), one molecule of hydrogen peroxide, one iodide ion and one hydrogen ion have been involved. That suggests that each of these appears in the rate equation. The other hydrogen ion and iodide ion don't appear until later. The mark scheme assumes that it must therefore be first order with respect to each. However, as I have tried to show on the Chemguide page, that isn't necessarily so. By the third stage (if that is the slow one), everything in the original equation has become involved. So on the same principle, it will be first order with respect to the hydrogen peroxide, but second order with respect to both hydrogen ions and iodide ions. However, if this is how CIE want you to look at it, why the reference to equilibria in the Examiner's Report? It doesn't make sense! I think this is just a badly thought-out question. I don't think it is our job to teach people to do problems in a way which is flawed, and will have to be un-learnt by students going on to do chemistry at a higher level. What to do about this? Don't worry about it! Given the lack of any detailed treatment in the new CIE book, if something similar gets asked again, most people still won't be able to do it. The only effect of that will be that grade boundaries will be lower on that paper. A question which nearly everyone finds too difficult just means that you will get an A, or whatever you want, with fewer marks. A hard question only matters if it is only you who thinks it is hard! Statement 8(g)(v) This statement wants you to be able to find an initial rate using data for change of concentration against time. The concentrations of the reactants will fall during the course of a reaction - so if you plot a graph of the concentration of one of the reactants against time, it will normally look something like this:
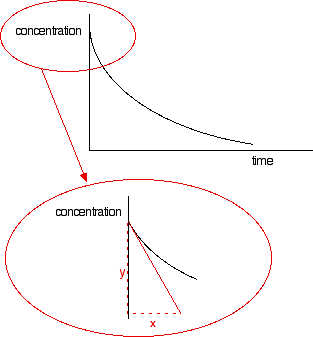
The steeper the slope of the curve, the faster the reaction. You can measure the slope of the curve by drawing a tangent to it. If you want the initial rate, then you draw a tangent right at the beginning of the curve, where it hits the concentration axis.
The slope of the curve at that point is found by drawing a triangle and measuring the values x and y. The slope is y/x. The units will be mol dm-3 s-1. If you have measured the time in some other units (such as minutes), these should be converted into seconds before you work out your initial rate. | |||||||||||||||||
|
Note: Technically, this slope should be given a negative value. This shows that the concentration of the reactant is decreasing. However, in rates of reaction work, we almost always tend to ignore this. | |||||||||||||||||
© Jim Clark 2011 (last modified August 2013) |
|||||||||||||||||