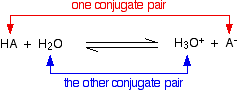|
Chemguide: Support for CIE A level Chemistry Learning outcome 7(h) This statement is about the Bronsted-Lowry theory of acids and bases. Before you go on, you should find and read the statement in your copy of the syllabus. You will find more than you need on the page about theories of acids and bases. Read the bit about the Arrhenius theory as background, although it isn't mentioned in the syllabus. Then concentrate on the section about the Bronsted-Lowry theory. You can ignore the long green box at the end of this, and you don't need to know about the Lewis theory either. Ignore that as well. The syllabus adds a comment about the "use of the acid-I, base-II concept". This is another way of talking about conjugate pairs, which are explained on the page you are about to look at. When you get to the section on conjugate pairs, you will find this equation:
Using the acid-I, base-II terminology:
If you wrote the reverse form of the equation, then presumably you would call H3O+ acid-I, and A- base-II. HA would then be acid-II, and H2O base-I To be honest, I have spent the whole of my adult life in chemistry education, and had never come across conjugate pairs being described in this way before. It doesn't seem to add much apart from some confusion!
© Jim Clark 2011 (last modified August 2013) |