|
Chemguide: Support for CIE A level Chemistry Learning outcomes 6(c), 6(d), 6(e), 6(f), 6(g) and 6(i) These statements deal with standard electrode potentials and their uses. I am dealing with these in one block for reasons I will explain below. Before you go on, you should find and read the statements in your copy of the syllabus. Just skim quickly through these to start with, and then come back to individual statements as you need to. Essential background work This topic can be a total nightmare. There are almost as many different ways of approaching this as there are teachers and writers, and most of these ways manage to make the whole thing completely confusing. Even the new A level Coursebook makes this feel more difficult and confusing than it is. CIE's syllabus and the teacher support material offer a sensible approach, but their exam questions and mark schemes up to the time of writing (May 2011) seem to take a different approach, and what they want sometimes seems inconsistent with the teacher support material. So what I would like you to do is to get comfortable with this topic without any reference to what the syllabus is asking point-by-point. Once you are comfortable, we will come back and look at the individual statements. On Chemguide, there is a set of five pages about this. They are intended to be read in order, and by the end of them you should have a clear idea of what is happening, and a simple visual way of using electrode potentials with confidence. The section starts with an introduction to redox equilibria, and you will find a link to the next page at the bottom of each page you finish. There are a few important points before you start:
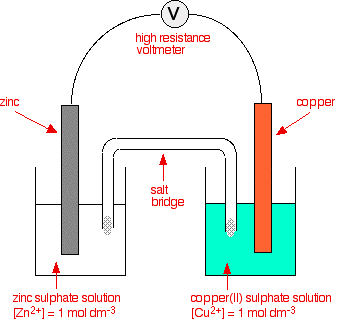
When you have finished this, you will have covered all of what the syllabus and the teacher support material asks, apart from some very simple calculations. It is now time to go through point-by-point to see how it all fits the syllabus, and to try to tie it into the sort of questions that get asked. Don't go on unless you are sure that you understand what you have read so far. Statement 6(c)(i) The definition of standard electrode potential is just over 3/4 of the way down the first page in the sequence you have just read. Don't forget to learn the conditions relating to the word "standard" from further up that page. Statement 6(d) This is about the standard hydrogen electrode. You will find that on the same page. Statement 6(e) You also will find the measurement of the standard electrode potentials of metals in contact with their ions on the same page. Measuring the standard electrode potentials of non-metals and their ions (such as chlorine and chloride ions) is discussed on the third page in the sequence, along with ions of the same element in different oxidation states. Statements 6(c)(ii), 6(f) and 6(g)(i) These statements are about overall cell potentials. The standard cell potential is the emf measured when you connect two half-cells together under standard conditions. The standard electrode potential is actually a special case of a standard cell potential where one of the half-cells is a hydrogen electrode. You can also imagine a cell made up of two half-cells like this:
The E° values for the two half-cells are:
The negative sign of the zinc E° value shows that it releases electrons more readily than hydrogen does. The positive sign of the copper E° shows that it releases electrons less readily than hydrogen. So you can work out that in the combined cell in the diagram, the zinc electrode will be relatively more negative, and the copper one relatively more positive. There will be more build up of electrons on the zinc than there are on the copper. Calculating the cell potential There is a very simple formula for doing this. To find the standard cell potential, you subtract the E° value of the left-hand half-cell from the E° value of the right-hand half-cell. E°cell = E°right - E°left So in this case: E°cell = +0.34 - (-0.76) volts = +1.10 volts Now, remember a convention that you came across early in the series of pages that you have read. The sign of E°cell when calculated in this way represents the sign of the right-hand electrode as you have drawn it. That means that the copper electrode (the right-hand electrode) is the positive one. We have already worked this out of course, but the calculation gives you another check on it. Suppose you had taken the same cell, but drawn it the other way around - with the zinc on the right and the copper on the left. If you had done the same sum, you would get an answer of -1.10 volts. Now that the zinc is the right-hand electrode, the answer shows that this is the negative electrode. The direction of electron flow in the external circuit This is from statement 6(g)(i), but just follows logically on from what we have been talking about. The two half-cells we have been using as an example are:
The position of the zinc equilibrium must be further to the left because it is the more negative. That means that there is a greater build-up of electrons on the zinc at equilibrium than on the copper. If electrons are allowed to flow through the external circuit, then they will obviously flow from where there are more of them to where there are fewer. They will flow from the zinc to the copper. Calculating the cell potential from a given equation If you are given a diagram of a cell, it is really easy to calculate the cell potential. If you are given an equation for an overall reaction, it suddenly gets much more confusing. A CIE question asked you to work out the E° for this reaction (although they made you work out the equation to start with!):
The first thing you have to do is to split the equation into two bits so that you can find the electron-half-equations, and the E° values which go with them. In this case, the Fe3+ ions are being converted into Fe2+ ions, and the Cu is being converted into Cu2+ ions. In an exam, you will be given a Data Booklet which will give you the relevant half-equations and their E° values. You can find that Data Booklet towards the end of the syllabus. E° values are listed twice - once in alphabetical order, and once in reverse electrochemical series order. Find these, and look at the list in alphabetical order. Now find the two equations you will need, and write them down exactly as they are listed in the table.
The two equations you should have found are:
Before we look at the calculation, just check that you can see why these values lead to the reaction we are interested in. The iron equilibrium is the more positive, and so will move to the right. The less positive (more negative) copper equilibrium will move to the left. That is exactly what the original equation is saying. Now all you have to do is to take one from the other in order to find the E° of the reaction. But there is obviously a problem here. Which do you take away from which? There isn't a right-hand and left-hand half-cell any more. For reasons which are beyond A level, the E° value of a reaction has to be positive. So when you do sums of this sort, you do the subtraction which leads to a positive answer. Make sure that you do this. The mark scheme for this question wouldn't allow an answer of -0.43 v. The answer had to be +0.43 v (or just 0.43 v). 0.43, of course, is what you get by subtracting 0.34 from 0.77. | |
|
Note: Being able to have either a positive value or a negative value in some circumstances, but only a positive one in others is quite confusing! You need to remember that getting a negative or a positive value by using the equation E°cell = E°right - E°left is simply a convention that quickly enables you to discover the sign of the right-hand electrode as you have drawn it. If you are interested in the voltage produced by a cell reaction, that will always be recorded as positive. | |
|
Statement 6(g)(ii) This statement asks you to make predictions about the feasibility of a reaction. You will already have read about this in the set of pages I asked you to look at at the start of this page. It would pay you to read about it again now. You will find it on this page. You will need the whole of this page, although you could leave the bit headed "Two examples where the E° values seem to give the wrong answer" until the next statement if you like. Statement 6(i) Start by reading the bit that I have just suggested you miss out in the last statement. What you are interested in is the second of the two examples where the outcome is influenced by the concentration of the reagents. Let's look at this again with a couple of simpler examples. We have discussed this copper half-equation before on this page:
For this E° value to apply, the copper(II) ions would have to have a concentration of 1 mol dm-3. What if the copper(II) ion solution was much more dilute? This is an equilibrium we are talking about. If you decrease the concentration of something in an equilibrium, the position of equilibrium shifts in such a way as to counteract the change (Le Chatelier's Principle). In this case, then, the position of equilibrium moves to the left, tending to increase the concentration of the copper ions again. But the E° value gives a measure of the position of the equilibrium. If the position of the equilibrium moves to the left, then the electrode potential becomes less positive. (It is no longer a standard electrode potential because the concentrations are no longer standard.) Suppose you have a half-equation involving two ions, such as
If you decrease the concentration of the two ions by the same amount, then it will make no difference to the electrode potential. Any tendencies for the equilibrium position to move will just cancel out. Horrible questions Students hardly ever find electrode potential questions easy, but in the two exam sessions in 2010, CIE came up with some really awful questions. Typically, the Examiner's Reports for these sorts of question blame the students for lack of knowledge and understanding, whereas the fault lies in unrealistic questions. These are extremely difficult to do under exam conditions unless you are really comfortable with the topic - and few students (and not all teachers either!) are really comfortable with electrode potentials. The question I am going to look at below comes from the May/June Paper 42 in 2010. This paper was so ridiculously hard that it was possible to get a grade A with 54% and pass with 28%. That means that even candidates worth an A grade couldn't do almost half of the paper.
I think this is disgraceful, and it isn't that uncommon on some CIE papers. The same grade boundaries also appeared on Paper 43 of that exam session, and on all the versions of Paper 4 set in the October/November exams that year it was possible to get a grade A with only 59%. In other words, good candidates still couldn't do about 40% of the paper. Anyway . . . The question I want to look at concerns the ease of oxidation of iron(II) compounds to iron(III) compounds under different conditions. This also relates to syllabus statement 9.5(h) in the transition metals section. It told you that Fe2+(aq) ions were relatively stable to oxidation by air under acidic conditions, but that a precipitate of iron(II) hydroxide is oxidised rapidly to iron(III) hydroxide under alkaline conditions. You were asked to use E° data from the Data Booklet to explain this. It was worth 4 marks. The Data Booklet is found towards the end of the syllabus. Read this next bit through with that beside your browser window on your screen if at all possible. Look first at the available equations involving iron. There are five of them in the alphabetical list of electrode potentials. The one involving the iron hydroxides is the last of them. You might worry that the equation seems to be showing a change from iron(III) hydroxide to iron(II) hydroxide, but this is a reversible change, and so that doesn't matter. The equation is
The only reaction involving simple iron(II) and iron(III) ions is:
You will have to use that one for the reaction under acidic conditions. Now you need to find reactions involving oxygen (from the air) under acidic or alkaline conditions. You will find three equations with oxygen on the left-hand side, but one of them ends up as hydrogen peroxide. Because there was no mention of this in the question, you would be safe to ignore this one. The other two versions have hydrogen ions in one of them, and hydroxide ions in the other - one under acidic conditions, and one under alkaline conditions. That's exactfy what you need. Under acidic conditions:
Under alkaline conditions:
Looking at these in pairs Under acidic conditions:
As a useful bit of revision before we go any further, notice that the oxygen equilibrium has the more positive E° value, and so will move to the right. The iron equilibrium will have to move to the left. That means that oxygen will oxidise iron(II) to iron(III) ions under acidic conditions. To make a comparison with the alkaline case, we need to work out the cell potential. Remember that the cell potential for a viable reaction has to be positive - so work it out by subtracting 0.77 from 1.23. That gives an answer of +0.46 volts. Under alkaline conditions:
Notice again that the oxygen equilibrium has the more positive E° value, and so will move to the right. Again, the iron equilibrium will move to the left, and so oxygen will oxidise iron(II) hydroxide to iron(IIII) hydroxide under alkaline conditions. So what's the difference from the other case? You need to work out the cell potential again. Again, you need a positive value, so E°cell = +0.40 - (-0.56) = +0.96 volts So the difference is that the cell potential is greater in the second case. A greater positive value for the cell potential means a greater likelihood of the reaction happening. And finally . . . So, could you do this under exam conditions in 4 minutes? On Paper 4, you should be working at almost exactly 1 mark a minute. If you had been taught to do this beforehand so that you had learnt exactly what you had to do, you might well get it right, but this set of reactions isn't mentioned specifically in any part of the syllabus or the teacher support material. You would have to work out how to do it as you went along in the exam. Few candidates could do that. Is there any point in learning it now? Probably not. CIE tend not to repeat their questions from year to year. It is completely impossible for me to go through every horrible question that CIE have ever set on this topic in this sort of detail. If you want to get ahead of other candidates in this topic, the only solution is to go through all the versions of Paper 4 that you can find, and look carefully at the mark schemes and Examiner's Reports. And then work away at it until you get a real feel for the sort of questions being asked, and how to answer them. But don't expect the same questions to come up again in future papers. Whether it would be worthwhile to spend time like this depends on how you feel about the topic. If you really hate it, it may be better to use your time elsewhere to polish up other parts of the syllabus. If possible, take advice from your teacher - he or she should know you best.
© Jim Clark 2011 (modified August 2013) |
|