|
Chemguide: Support for CIE A level Chemistry Learning outcomes 4(f) and 4(g) These statements are a brief introduction to ceramic materials. Before you go on, you should find and read the statements in your copy of the syllabus. The IUPAC Gold Book defines ceramic as
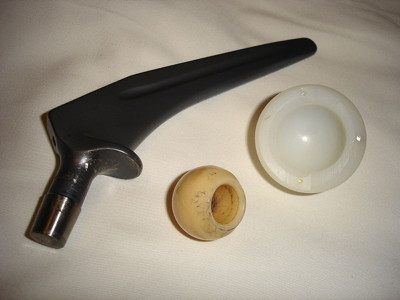
In the first sentence, the word "sintered" refers to the process of sintering. Sintering happens when you heat a powdered material to a temperature below its melting point, and new bonds are formed between the grains of powder to form one large mass. The most commonly used definition (including by CIE) tends to include what the note says. There are several modern ceramic materials which don't actually involve a metal. The syllabus, for example, includes silicon(IV) oxide, and there is a past question which tells you that silicon carbide, SiC, is used in ceramics, and asks you to deduce things about it. This is such a huge topic that I will just stick closely to what the syllabus asks. Statement 4(f) This deals with the properties of some ceramic materials, and talks about the physical properties of ceramics in terms of their structures. The structures I have a problem with this, because I disagree with the syllabus statement! This says ". . . in terms of their giant molecular structure". A giant molecular structure (I prefer the term giant covalent structure) consists of covalent bonds holding together huge numbers of atoms. If you have been working through previous statements in Section 4, you will already have read about these in learning outcome 4(e)(iii). The problem is that some of the ceramics mentioned in 4(g) aren't covalent. Magnesium oxide is ionic, and aluminium oxide is also mainly ionic. They do indeed have giant structures, but they have giant ionic structures. Other more complex ceramics will contain both ionic and covalent bonds, some of which may be highly polar. What is, however, true is that ceramics all have giant structures of one type or another, with strong bonds between the atoms (or ions) which make them up. The physical properties Strength The strong bonds holding the atoms (or ions) together in three dimensions will make the ceramic hard and strong, but also brittle. Think about a ceramic wall or floor tile as a simple example. The tile is obviously hard, and you can stand on a floor tile, or place heavy furniture on it without it breaking - so it is strong. On the other hand, it isn't difficult to break a tile in half by snapping it, especially if you score a shallow line on it first with a cutting tool - it is brittle. This is unlike a metal, which would bend before it fractured - you can bend or stretch metals. Atoms in metals can roll over each other without breaking the bonds. In a ceramic, you either have to break covalent bonds in three dimensions or, if you distort an ionic lattice, it brings like charges together, which splits the lattice apart. The lattice is either all in one piece, or else broken apart. Melting points Ceramics have high melting points because of the need to break the strong covalent or ionic bonds holding the giant structure together. It takes a very high temperature to do this. Electrical conductivity Most ceramics are good electrical insulators. If they are covalent, there are no free electrons to move around. If they are ionic, the ions aren't free to move in the solid. Examples of electrically conducting (including superconducting) ceramics are beyond the scope of this syllabus. Statement 4(g) This deals with the uses of modern ceramic materials involving magnesium oxide, aluminium oxide and silicon(IV) oxide, and the relationship with their structures. Magnesium oxide Ceramics made from magnesium oxide are used in (amongst other things):
Aluminium oxide Various web sources describe this as the most important oxide ceramic. Uses of aluminium oxide ceramics include:
Silicon(IV) oxide (silicon dioxide) Uses of silicon dioxide ceramics include:
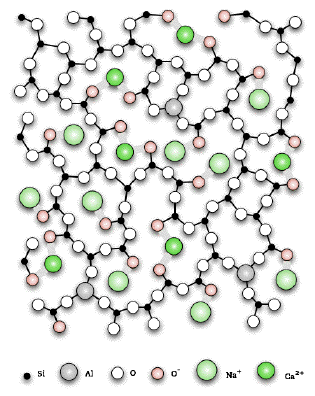
You will notice that I haven't specifically quoted two of the uses suggested in the syllabus - the use in glass and crockery. The reason is that although silicon(IV) oxide, for example, is used to make glass, the resulting glass isn't pure silicon(IV) oxide. Instead, it will be a complex lattice with (mainly) ionic bonds to various other metal ions present. This diagram (also taken from Wikipedia) shows a possible structure for a particular glass.
To describe this as silicon(IV) oxide would be chemical nonsense! It is possible to make a ceramic containing pure silicon(IV) oxide which looks like glass, but it isn't normal glass. The only times I ever came across it were in a chemistry lab. The problem with pottery is even worse. Various kinds of pottery are made by heating to high temperatures various clays or mixtures of clay with other things. The results are chemically very complex. It would be completely misleading to describe pottery as being made of any of the ceramic materials mentioned in this syllabus statement. | |
|
Note: Up to the November 2012 papers, all you needed to do was to identify "baked clay found in crockery" as containing a giant structure. You just had to recognise baked clay as a ceramic, and know that ceramics had giant structures. You didn't need to know what that structure was. | |
© Jim Clark 2010 (modified August 2013) |
|