|
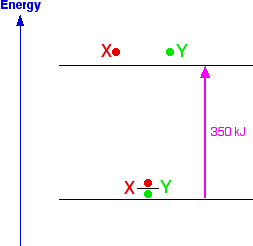
Chemguide: Support for CIE A level Chemistry Learning outcome 3(n) This statement is an introduction to the idea that energy changes during a reaction are caused by bond breaking and making. Before you go on, you should find and read the statement in your copy of the syllabus. This is dealt with numerically in Section 5, and this current page is only intended to give a brief introduction. Chemical reactions involve breaking the bonds in one set of substances, and re-organising them to make new bonds in new substances. Breaking bonds needs energy That's fairly obvious really. Whatever sort of bond you are talking about, it involves one thing being attracted to something else. To separate them, you are going to have to put in some energy. Making bonds releases energy That's not so obvious. Whenever you set up an attraction of any sort, energy is released. Think of it like this. Suppose you have a covalent bond between two atoms X and Y. To split them into the two individual atoms takes, say, 350 kJ for a mole of bond. You can show this happening on an energy diagram.
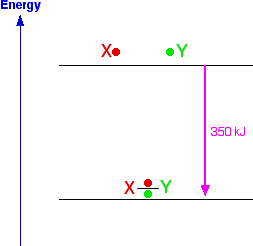
Nows suppose you remade the bond from the same atoms. Obviously, you would be exactly reversing what you had just done in breaking the bond, and will end up exactly where you started. That means that you will get out exactly the same amount of energy as you put in to break the bond.
Exothermic and endothermic changes In any reaction, you may well be breaking several bonds, and so you will need to put in enough energy to do this. Then you will make a number of bonds and, at that stage, you will get energy released again. Why are some reactions exothermic? Suppose you need to put in 1000 kJ to break all the necessary bonds in your starting substances. Suppose also that the new bonds formed are stronger than the original ones, so that you get 1200 kJ of energy released as the new bonds are made. You get out 200 kJ of energy more than you put in. That's an exothermic reaction - one which releases the extra energy as heat. Why are some reactions endothermic? Suppose you need to put in 1000 kJ to break all the necessary bonds in your starting substances. Suppose this time that the new bonds formed are weaker than the original ones, so that you only get 900 kJ of energy released as the new bonds are made. You put in 100 kJ of energy more than you get out. That's an endothermic reaction - one which absorbs heat energy.
© Jim Clark 2010 (modified July 2013) |