|
Chemguide: Support for CIE A level Chemistry Learning outcome 3(i) This statement introduces bond energies, bond lengths and bond polarities, and looks at the relationship between them and the reactivity of a particular bond. Before you go on, you should find and read the statement in your copy of the syllabus. I see this as only an introductory topic at this point. You will meet examples relating to this throughout the course, in both inorganic and organic chemistry. The examples I have discussed lower down this page aren't meant to be learnt at this stage. Bond energy Bond energy is a measure of the strength of a particular covalent bond. In fact, it is sometimes referred to as "bond strength". You may find bond energies also referred to as bond enthalpies. There is a subtle difference between the two terms, but they are often used as if they mean the same thing. Bond energies in simple diatomic molecules Diatomic molecules are ones which contain two atoms. For a simple covalent molecule, X-Y, bond energy is the amount of energy needed to break one mole of the covalent bond to produce individual atoms, starting from the original substance in the gas state, and ending with gaseous atoms. X and Y can be the same or different. So, if they are the same, for example in chlorine gas, it is the energy needed to carry out this change per mole of bonds:
The bond energy of the Cl-Cl bond is +244 kJ mol-1. That means that it takes 244 kJ to break 1 mole of Cl-Cl bonds. | |||||||||||||||||
|
Note: Don't worry if the units aren't familiar for now. You can just take the size of the number as a way of comparing bond strengths. As far as the values are concerned, I am using those from the CIE Data Booklet as far as possible, because that's what you will get in an exam. You will find a copy of this towards the end of the syllabus. The values I give for bond energies in this CIE part of Chemguide might be 1 or 2 kJ different from values I have used elsewhere on Chemguide, because those came from a different source. | |||||||||||||||||
|
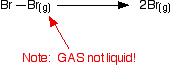
And for bromine, the reaction is still from gaseous bromine molecules to separate gaseous atoms.
In this case, the bond energy is +193 kJ mol-1. That means that the Br-Br bond is weaker than the Cl-Cl bond. If the atoms are different at each end of the bond, it works in exactly the same way. For example, the bond energy of the H-Cl bond is +431 kJ mol-1, and is given by the equation:
You have to be very careful about this. Bond energies only work if you are going from the substance in the gas state to gaseous atoms. So what if you have liquid bromine, for example? There will be extra energy needed to first convert the liquid into the gas. Similarly, in a real reaction, you aren't going to end up with gaseous atoms - they will react with other things, and there will be other energy changes there as well. In chemistry, it is often helpful to break complicated processes down into small steps. You will come across this a lot in the future. Bond energies in more complicated molecules The bond energy of the C-H bond, for example in methane, CH4, is quoted by CIE as 410 kJ mol-1. However, if you took methane to pieces one hydrogen at a time, it needs a different amount of energy to break each of the four C-H bonds. Every time you break a hydrogen off the carbon, the environment of those left behind changes. And the strength of a bond is affected by what else is around it. In cases like this, the bond energy quoted is an average value. In the methane case, you can work out how much energy is needed to break a mole of methane gas into gaseous carbon and hydrogen atoms. That comes to +1640 kJ and involves breaking 4 moles of C-H bonds. The average bond energy is therefore +1640/4 kJ, which is +410 kJ per mole of bonds. That means that many bond energies are actually quoted as average bond energies, although it might not actually say so. In fact, tables of bond energies give average values in another sense as well. The bond energy of, say, the C-H bond varies depending on what is around it in the molecule. That means that if you use the C-H value in some calculation, you can't be sure that it exactly fits the molecule you are working with. When you come to doing simple calculations with bond energies later on in the course, you have to remember that the answers you get are only approximations to what happens in real reactions. Bond length Bond length measures the distance between the two nuclei in a covalent bond. The important thing about bond length is its relationship with bond energy. In a covalent bond, the two atoms are held together because both nuclei are attracted to the same pair of electrons. In a longer bond, the shared electron pair is further from at least one of the two nuclei, and so the attractions are weaker. For example, in the hydrogen halides, as the bond gets longer, the distance of the electron pair from the nucleus of the halogen atom is going to get much greater, and that weakens the bond.
The short H-F bond is very strong, and that has a major effect on the chemistry of fluorine. The bonds then get weaker as they get longer as you go down the Group. You will find examples of these effects in the chemistry of these elements when you do some Group 7 chemistry later. Bond polarity If you are working through these Section 3 syllabus statements in order, you should already have read the page about electronegativity in the bonding section of Chemguide. You need to be sure that you understand what electronegativity means and what causes electronegativity differences. And of course, you must know what is meant by a polar bond. A polar bond is one in which two atoms of unequal electronegativity share a pair of electrons. The electrons are pulled towards the atom with the greater electronegativity. That leaves the more electronegative atom slightly negatively charged and the less electronegative one slightly positively charged. What does all this mean for bond reactivity? When a reaction happens, some bonds are broken and new ones are made. Obviously bond energies will play a major part in this. But in many cases, the reaction is triggered by some sort of attraction between two molecules. This is where the polarity of the bonds plays a part. To illustrate this, I would like you to look at a page in the organic chemistry section of Chemguide about nucleophilic substitution reactions. Just read down as far as the red warning notice. Don't worry if this is all unfamiliar. All I am trying to illustrate is that when you look at a reaction in this sort of detail, you have to consider both bond polarities, and bond energies. In this particular case, the success of the reaction is determined by the strength of the carbon-halogen bond, and not the polarity of the bond. And here is another example, also from organic chemistry. Have a look at a page about electrophilic addition reactions. Remember that you aren't trying to learn this at the moment. I just want to give you an impression of the way in which we can explain how reactions happen, and the role of bond polarities in this. Now look at a page about the reactions of ethene with hydrogen halides. Ignore any mention of cyclohexene, and concentrate on the section titled "Electrophilic addition reactions involving the other hydrogen halides". Notice that, again, what determines how fast the reactions happen is the strength of the hydrogen-halogen bonds, not their polarity. Hydrogen fluoride is the most polar molecule, but the reaction is the slowest because of the difficulty of breaking the hydrogen-fluorine bond. And one more example: Hydrogen and chlorine react to make hydrogen chloride. Hydrogen and iodine react to make hydrogen iodide. In each case, you have to break the H-H bonds in the hydrogen gas, and the halogen-halogen bonds in the chlorine or iodine. The bond energies for the two halogens are:
So which is the more reactive halogen, chlorine or iodine? Well, if you look at the bond energies, it is much easier to break iodine-iodine bonds than chlorine-chlorine bonds. So you might expect the iodine to react faster. Not so! In the presence of a flame or bright sunlight, chlorine and hydrogen react together explosively. On the other hand, you have to heat hydrogen and iodine together continuously, and even then only a proportion of them actually react. By contrast with the chlorine case, the hydrogen and iodine reaction is really feeble. The problem is that the bond energies of the halogens are just one factor in the overall process, and you need to look at the strengths of the bonds being formed as well. This is a problem for later on in the course. I just wanted to point out at this stage that you can't safely draw any conclusions from what happens in just one step of a complicated series of reactions. Summary Bond length plays a part in the reactivity of a covalent bond because it affects bond strength. Bond polarity affects reactions because it can help in getting the molecules attracted and lined up in the right way. In reactions involving covalent molecules, bond energies will play a part in determining reactivity, but you can't make assumptions about this just by looking at one stage of the overall process. | |||||||||||||||||
|
Note: I worry about this learning outcome being included at this early stage of the syllabus. There is a real danger of making over-simplifications in order not to scare students too much. Explaining reactivity really has to be done on a case-by-case basis. But there is hope! In the six sets of exam papers, plus the specimen paper, that I had available at the time of writing (June 2010), I couldn't find a single question which related directly to this statement. | |||||||||||||||||
© Jim Clark 2010 (modified July 2013) |
|||||||||||||||||