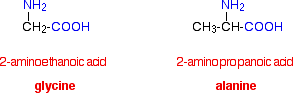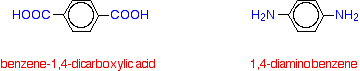|
Chemguide: Support for CIE A level Chemistry Learning outcome 11.3(d) This statement is about the way that side-chains and intermolecular forces affect the properties of polymers. Before you go on, you should find and read the statement in your copy of the syllabus. The effect of side-chains in polythene I want to start by considering the effect of simple side chains in addition polymers. You should read about high and low density polythene on the page about the polymerisation of alkenes. You don't need to worry about the way they are manufactured. Just concentrate on how the presence of side chains affects their physical properties. The difference between the structures of LDPE and HDPE in relation to their densities and melting points was asked in a question in June 2012. There was enough information in the question to enable you to draw suitable diagrams, and you could think out the other parts of the question. It would be easier if you had some previous knowledge though. Spider silk Spiders produce several sorts of silk, but the one we are interested in is used for the web and is known as "dragline" silk. This is both very strong and very ductile (able to be extended without breaking). It is made from a protein containing high proportions of glycine and alanine residues.
The alanine residues tend to be found in what are known as "crystalline" regions of the protein, arranged in very regular beta-sheets. If you have forgotten about beta-sheets, remind yourself about them on the page about protein structure. Just read the bit about beta-sheets. The beta-sheets are, of course, held together by hydrogen bonds. The glycine residues are found in "amorphous" regions of the protein. These regions contain various other structures including alpha-helixes, and spirals based on short lengths of glycine residues. Hydrogen bonding is involved in these as well. These spirals can be stretched out if the silk is pulled - rather like very loose springs. When the tension is released, the spirals can reform, and so the silk can return to its original length. A material which can do this is said to be elastic. So you can think of spider silk as being a composite material containing regions which are very strong, and others which are very elastic. There has been a half of a question (worth 5 marks) asked about this in the first 13 exam sessions of the current syllabus. It gave you diagrams comparing spider silk with silk from silkworms, and asked you what sort of bonding existed between adjacent parts of the protein chains. Hydrogen bonding was acceptable for both forms of silk. It then went on to ask for two differences in properties between the two types of silk. The diagram for silkworm silk showed that it had a higher proportion of more closely packed beta-sheets, and less of the amorphous regions than there are in spider silk. Two easy answers would be:
The final mark told you that the spider silk contained large amounts of residues from glycine, and asked how this affected the properties of the silk. You obviously had to know that glycine residues make up an important part of the structure which is responsible for the elasticity of the silk. So all you needed to say was that it makes the silk more elastic. Kevlar Kevlar is a very tough polyamide. It is made by polymerising benzene-1,4-dicarboxylic acid and 1,4-diaminobenzene.
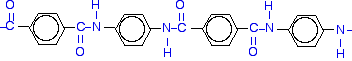
If you line these up and remove water between the -COOH and -NH2 groups, you get the structure of Kevlar:
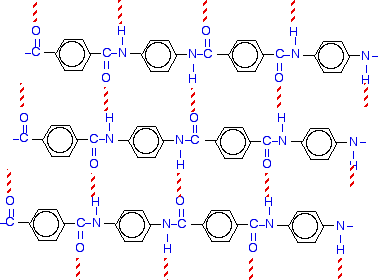
What is interesting about it from the point of view of this particular syllabus statement is the way that the molecules can line up to form strong sheets. The bonding between the individual strands of Kevlar is hydrogen bonding. The next diagram shows this for three strands of Kevlar. The red dotted lines represent hydrogen bonds.
Make sure you understand what is going on. A past question asked you to do just this - draw a second strand to show how it was attached to one drawn in the exam question. November 2008 paper 4 Q10 The was part of a question which started off with spider silk. The question ended by asking you to suggest why condensation polymers like proteins show a wider range of properties than addition polymers. This is one of those questions where it is very difficult to guess what the examiner is looking for. That is why I am talking about it specifically now. It is probably unlikely to come up again, but it might give you some hints as to how to answer a similar question in the future. The mark scheme wanted any three of the following points. In fact, it is easiest to concentrate on the variable properties of proteins, but you mustn't forget to compare them with addition polymers as well. This is my version of what the mark scheme wanted - trying to pull it into a logical sequence!
© Jim Clark 2011 (modified August 2013) |