|
Chemguide: Support for CIE A level Chemistry Learning outcome 11.3(c) This statement is about polymerisation - addition and condensation. Before you go on, you should find and read the statement in your copy of the syllabus. This is really just a straightforward repeat of what you will already have met in the syllabus. I suggest that you just work through the section 10.8 menu as a useful revision. You need to have a good understanding of polymerisation. You may be lucky enough to have a question which talks about familiar polyesters or polyamides or common addition polymers, but you could be faced with completely unfamiliar cases. If that happens, you should be able to work the structure of a polymer from its monomer or monomers. In June 2008 paper 4 Q10(c), you were given three monomers and asked to draw a polymer which could be made using just one of them, or some combination of 2 of them, or possibly using all of them. You had a choice of what you used in this question, and you could make straightforward polymers such as a polyester or a polyamide. However, I want to just take one of the monomers, and see how you could make a polymer from that. The monomer you had to work from was:
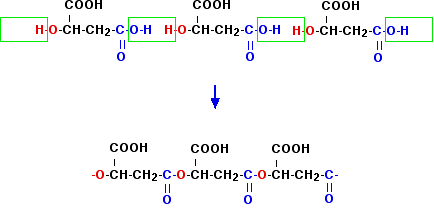
When you normally draw monomers, you draw them so that you can easily see how they join up. That isn't so in this case, which is why I have chosen it. I assume you can do the easy ones! So how do you work out what happens? Can this form an addition polymer? No - it doesn't have a C=C double bond. The only condensation polymers you know about involve the formation of polyesters or polyamides. It obviously can't be a polyamide you are looking for because there is no nitrogen in the molecule. What about a polyester? There is in fact an -OH group tucked away in the middle of the molecule - so the first thing to do is to redraw the molecule to give this more importance. You need to draw the molecule to show a -COOH group on one end and an -OH group on the other. There are actually two ways you can do this, depending on which of the -COOH groups you want to concentrate on:
The reason for the colour-coding is to pick out the two groups in each molecule that we need to concentrate on to make a polyester. Before you go any further, convince yourself that these are exactly the same molecule, and are also exactly the same as the formula further up the page. For example, in the left-hand one, start from the -COOH group at the top and trace your way through the molecule so that you can see that it is all joined up in the same way as the one above. All that has happened is that these molecules have been bent and coloured to make their structure easier to see for polymerisation purposes. I'm just going to produce a polymer from the left-hand one of these. Make sure you understand what is going on by doing the same thing to the right-hand one.
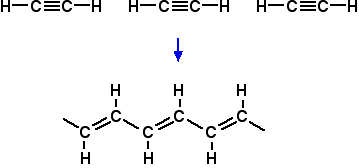
In the exam, either this polymer or the one you get from the right-hand structure was allowed. This all looks a bit scary, but if you can take your time over it, you get there in the end. But whether you would have the time and patience to get this right in an exam is another matter altogether. In fact, in the exam question this came from, there was a much simpler choice you could have made. As I have said before in this part of the syllabus, please don't waste time learning this example - it is very unlikely to come up again in exactly the same form. Make sure that you understand polymerisation well enough to be able to work it out if you need to. Thermoplastic and thermosetting polymers In November 2011 papers 41 and 42, CIE asked a three mark question about thermoplastic polymers, despite the fact that the term isn't mentioned in the syllabus or in any of the support material. I can't find it in the Coursebook either. If you hadn't come across the term, the question is impossible to answer. A clearer term to use than "thermoplastic" is "thermosoftening". If you talk about thermosoftening plastics and thermosetting plastics, it suddenly becomes much clearer what you are talking about. Remember: thermoplastic = thermosoftening Thermosoftening plastics are ones which soften, and eventually melt, on heating. Typical examples include poly(ethene), PET (poly(ethylene terephthalate) - a polyester) and nylon. These plastics can be re-melted after use and shaped into new forms. For example, PET is used for drinks bottles to hold mineral water, soft drinks, and so on. After recycling, the plastic can be remelted and spun into fibres to make clothing when it would now be called polyester. The reason they soften and melt on heating is that the eventually enough energy is supplied to overcome the intermolecular attractions between the long chain molecules, and they become free to move around to some extent. Thermosetting plastics are quite different. In the production of thermosetting plastics, covalent bonds are made between the original polymer chains to give a cross-linked structure which is essentially one huge molecule. Because covalent bonds have to be broken to allow anything to move, much more heat has to be supplied, and this tends to break down the whole structure into random bits. Instead of softening and then melting on heating, thermosetting plastics tend to char (blacken), but remain solid. Examples of this include Bakelite (one of the first plastics) and epoxy resins. You couldn't recycle one of these materials to use for something else. A further comment on the November 2011 question The question asked you to say which type of polymerisation produces thermoplastic polymers, and the mark scheme gave the answer "addition polymerisation". This implies that only addition polymers are thermoplastic (thermosoftening). That is simply untrue. PET (mentioned above) is a condensation polymer, as is nylon, which also melts on heating. The question is not only unfair because it isn't mentioned by CIE anywhere in their syllabus or support material, but is also a question that can't meaningfully be asked. Both types of polymerisation (addition and condensation) can produce thermoplastic polymers. Polymers which conduct electricity There is no mention of these polymers in the syllabus. They aren't mentioned by the Chemistry Coursebook. But in the second version of the Application Support Booklet, in the section about what the student needs to know, it mentions the formation of such polymers. There was a part question in June 2012 in which you were given a structure for such a polymer, and asked straightforward questions about it. I will just give you enough information so that you can recognise the possibility that a given polymer can conduct electricity. Anything more than that is way beyond A level chemistry. If you polymerise ethyne (acetylene), this happens:
What you need to notice are the alternating single and double bonds in the polymer. That continues the whole way along the chain. You should remember that when carbon forms a double bond, one of its p orbitals is at right angles to the plane of the molecule, and that these overlap sideways to give the pi bond in, say, ethene. That is happening to every carbon atom in this chain, and all of the p orbitals can overlap sideways to give a delocalised system of electrons the whole length of the chain. The presence of the delocalised electrons over the whole chain is what enables it to conduct electricity. If you are given any polymer structure where you can trace alternating single and double bonds down the whole chain, then it could have the ability to conduct. In truth, it isn't so obvious why delocalisation along a single chain can allow the material in bulk to conduct, but as I said before, that is way beyond the sort of knowledge you need for this exam. The June 2012 question had alternating benzene rings and C=C double bonds. You had to recognise the delocalisation of the electrons, and you were also asked about the geometry of the molecule which enabled it to conduct. The point here was that it had to be planar, because otherwise the p orbitals on the benzene rings wouldn't line up with those in the C=C bonds.
© Jim Clark 2011 (last modified August 2013) |