|
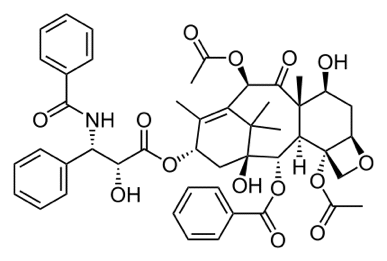
Chemguide: Support for CIE A level Chemistry Learning outcome 11.3(a) This statement is about the design of drugs. Before you go on, you should find and read the statement in your copy of the syllabus. Background There are a number of areas that you should be aware of. Natural and synthetic drugs Taxol The anti-cancer drug Taxol was originally obtained from the bark of the Pacific yew tree. Unfortunately, it occurs in such small amounts that it was estimated that you would have to cut down six 100 year-old trees to get enough Taxol to treat just one patient. Clearly that is impossible, and so a lot of work went into finding a way of synthesising the drug in the lab. Because Taxol is a seriously complicated molecule, that wasn't so easy. However, eventually it was done. Here is the structure of Taxol shown as a skeletal formula. The dotted bonds are going back away from you, and the solid wedges are coming out towards you. More about that later.
| |
|
Note: You will also find this used as an example in the Application Support Booklet, but the structure is wrong in both the first and second versions of the Booklet. In this instance, that doesn't matter particularly, because you don't have to know any detail about this. It has appeared in a CIE question, though, and that version was correct. | |
|
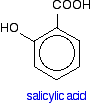
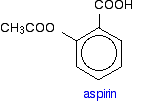
Aspirin Sometimes, natural products need to be modified to reduce side effects. The discovery of aspirin is a simple example of this. The bark of willow trees had been used for a long time as a remedy against fever and pain. The active ingredient, salicin, was found to contain salicylic acid.
Unfortunately, salicylic acid causes serious mouth irritation. The molecule was then modified to make it safer, but without losing any of its helpful properties. The result was aspirin - chemically known as (using its common older name) acetylsalicylic acid.
How are complex structures like Taxol worked out? You have already come across the two main ways of working out the structures of complex molecules in learning outcome 11.2(e). These are X-ray crystallography and NMR. Go back and look at this again - particularly the summaries of what each technique tells you. You don't need to read about MRI and medical uses again. The importance of the shapes of drug molecules Many drugs act as enzyme inhibitors. If you aren't sure what this means, then you really need to go back and revisit the Chemguide pages about enzymes. If you do this, concentrate on the lock and key mechanism for enzyme catalysis on the first of the three pages, and inhibitors on the final page. Aspirin blocks the action of an enzyme involved in the production of molecules called prostaglandins. These are important in the transmission of nerve signals (including pain signals) amongst other things. The enzyme has its active site at the end of a "tunnel" in the enzyme's tertiary structure. Aspirin reacts irreversibly with groups near the outer end of the tunnel, blocking other molecules from reaching the active site. Aspirin is therefore an example of a non-competitive inhibitor. Ibuprofen, another painkiller, works differently. This is a competitive inhibitor. It has the right shape and arrangement of groups to be able to fit into and bind (reversibly) to the active site of the enzyme. It just gets in the way. Both aspirin and ibuprofen block the enzyme's action, but in different ways. It is important to realise that you don't have to remember any of this! If you are ever asked about the action of any drug, you will always be given enough information to work with. What you would be expected to do is to relate what you are told to your knowledge about enzymes, active sites, inhibition, and the various sorts of bonding that might be involved in all this. You mustn't get the idea that drugs only target enzymes. As a non-biologist, I don't understand how Taxol works as an anti-cancer drug, except that it interferes with cell division in a way that doesn't involve enzymes. In November 2009 paper 41 Q 9, CIE asked about two anti-cancer drugs which bound to DNA and interfered with the way it replicated. In this case, you would have had to remember something about the way that DNA strands were bound together, and the need for the individual strands to "unzip" either in producing new DNA or making RNA. Drug design using computers Traditionally, making new drugs or improving old ones was a matter of making huge numbers of different compounds and then testing them - a time-consuming and expensive process. Computers have made this much more efficient. You can produce accurate 3-dimensional models of a possible drug molecule and of the biological site that you want it to attach to - the active site of an enzyme, for example. That way you can design molecules which will have exactly the right shape and the right arrangement of groups to fit the site exactly, and all before you have to do any testing. You still have to test, of course. The molecule you have designed might have other problems. For example, its solubility might not be right, or it might cause unexpected side effects. Chiral drug molecules If you can't remember what chiral molecules are, then go back and look again at learning outcomes 10.1(f),(h) and (i). The Taxol molecule has a lot of chiral centres. These are carbon atoms which have four different groups attached to them. You can spot them very easily in this particular diagram of the structure of Taxol, because these are the ones which show the spatial arrangement of the atoms using either dotted or solid wedges.
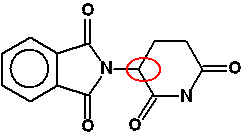
Make sure that you understand why these all have four different groups attached to a carbon atom. Don't forget that in a skeletal structure, there is a carbon atom at each junction between two or more lines, and one at the end of a line. For example in the bottom right-hand corner of the diagram, there is a carbon at the right-hand end of the line: a CH3 group. If there aren't four lines leaving any carbon atom, then there are enough hydrogens attached to bring the total number of bonds up to four. It hasn't been asked yet (up to June 2013), but CIE could well give you a simpler structure than Taxol, and ask you to draw a circle around any chiral centres. So why is the presence of chiral centres important to drug design? Each chiral centre will have two possible arrangements of bonds around it. A different arrangement of bonds will mean a differently shaped molecule. And differently shaped molecules won't necessarily fit the active site of an enzyme, or whatever else the drug needs to attach to. A fairly simple example of a drug with a single chiral centre is thalidomide - a drug which was prescribed for morning sickness in pregnancy in the 1950s. The chiral centre is circled in red below:
This will have two optical isomers. One was the right shape and so did what it was supposed to, but the other one behaved completely differently, causing major birth defects. It is believed that the isomer responsible interfered with DNA. These days, a lot of effort goes into producing a single isomer of a drug. Chemical reactions frequently produce mixtures of optical isomers. Trying to produce just one isomer is known as asymmetric synthesis. Reasons for producing drugs consisting of a single active isomer:
Questions you might get asked It is important to say again that you don't need to learn any of the detailed stuff above about particular drugs. If they ask you about a drug, its structure and other necessary information will be given you in the question. Very few questions had been asked about this statement up to the June 2013 exam session, but in general terms, they are likely to be of two sorts. Questions where you need some knowledge of chemistry You could easily be asked about the way a drug interacts with an active site on an enzyme in terms of its shape and the groups attached. That means that you would have to be familiar with the lock-and-key description of an enzyme and its substrate, and the sorts of bonding which occurs between them (ionic, hydrogen bonding and van der Waals). You would also need to be familiar with enzyme inhibition. A question I mentioned above did something similar with drugs attaching to DNA. That means that you will need to know about the replication of DNA or the formation of RNA. Shapes of molecules are important here, and you could be asked to recognise chirality in a drug molecule. You could also be asked how the structures of these complex molecules are worked out. You need to know about X-ray crystallography being used to map the arrangement of the main atoms, and NMR to tell you about the environments of the hydrogen atoms. There is nothing new that you need to learn in this. It is all part of the chemistry you should already know. Non-chemistry questions These are questions which rely on general knowledge or common sense, and it is often difficult to know exactly what the examiners are looking for. In fact, what they want is often fairly trivial. For example, you could be asked:
These questions are just quick examples of what you could get asked, but they won't be the only things. Neither, I suspect, are the suggested answers the only possible ones. All this is intended to do is to give you some sort of idea of how you might think about questions of this kind.
© Jim Clark 2011 (modified August 2013) |
|