|
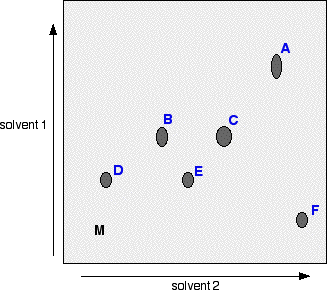
Chemguide: Support for CIE A level Chemistry Learning outcome 11.2(g) This statement is about various sorts of chromatography. Before you go on, you should find and read the statement in your copy of the syllabus. You will find everything you need if you work through all the pages from the chromatography menu. It is important that you work through these in the order listed in the menu, and not in the order given in the syllabus statement. You might think that it is easier to start with something simple like paper chromatography, but there are problems with explaining paper chromatography. Thin layer chromatography: TLC So start with thin layer chromatography. This is just like the paper chromatography you may be familiar with, except that you use something other than paper. Make sure that you fully understand why some things move further than others during the chromatography. Column chromatography The next page from the menu is about column chromatography. This isn't mentioned by the syllabus, but is a useful bridge between thin layer and high performance liquid chromatography. It is easy to understand, and will make HPLC look less scary. You don't need to remember any details about this, of course. High performance liquid chromatography: HPLC There are two types of HPLC. The Applications Support Booklet mentions this in passing, and the Chemistry Coursebook seems to be describing reversed phase HPLC, but without actually calling it that. I suggest that you concentrate on reversed phase HPLC, and ignore the other form. If you try to learn both forms, you are only going to get confused. Make sure that you understand what is going on in the column, because that is what is most likely to be tested. Gas-liquid chromatography In gas-liquid chromatography, you should again concentrate on what is happening in the column so that you can explain why some things pass through faster than others. Again, that is the most likely thing for you to get tested on. A past question (November 2010 paper 43 Q10) gave you an output chart with two triangular shaped peaks labelled X and Y, and asked you to work out the percentage of each of X and Y in the mixture. What you needed to do was to find the areas under the two peaks, add them together, and then work out the percentages. To do that, you need to remember a simple bit of geometry - the formula for the area of a triangle. That's 1/2 x base x height. You needed to take measurements from the exam paper. In fact, in this case, the bases of the two triangles were identical, and so you could take a short-cut, and just work from the heights if you had enough confidence. Paper chromatography Finally, you can return to the more familiar paper chromatography. You will find that much of this is a repeat of the thin layer chromatography you started with. CIE count the mechanism for paper chromatography as partition of the solutes between the solvent and water trapped in the cellulose fibres. Although I think you should read about the problem with this explanation in the final section of the page, don't try to remember it. If you are ever asked for an example of paper chromatography, just avoid the use of water (or any water-soluble solvent such as alcohol) as a solvent. Be sure that you understand two-way paper chromatography. This was asked four times in the first 13 exam sessions of the current syllabus. You must be able to interpret a 2-way chromatogram. For example, suppose you were given a 2-way chromatogram with a set of seven spots (labelled A to F) like this:
M represents the position of the original drop of mixture. Can you answer questions like these? How many spots would there have been in the chromatogram after you had used solvent 1 and before you used solvent 2? 4. B and C wouldn't have separated out, and neither would D and E. Which spot is due to a substance almost insoluble in solvent 2? D - because it has hardly moved at all when solvent 2 was used? Which spot is due to a substance almost insoluble in solvent 1, but very soluble in solvent 2? F - because it only travelled a little way up the paper when solvent 1 was used, but was carried a long way by solvent 2. Partition or adsorption? You must be sure that you can define partition and adsorption in the context of chromatography. Partition chromatography is a separation due to the different solubilities of a solute in two different solvents - one the mobile phase and the other the stationary phase. Paper chromatography and gas-liquid chromatography both involve partition. If you aren't sure about this, go and read those pages again. Adsorption chromatography is a separation due to molecules having different attractions for the solid stationary phase and the liquid mobile phase. If there are strong attractions between the solute and the stationary phase, the solute won't move very far - it will spend most of its time sticking to the stationary phase. On the other hand, if there are weak attractions between the solute and the stationary phase, but strong attractions between the solute and the solvent, it will spend most of its time in solution, and so travel much further. Thin layer chromatography involves adsorption. What about HPLC? This depends on which method you are talking about. If you are talking about the most commonly used form (reversed phase HPLC), it is debatable whether it involves partition or adsorption. CIE have sensibly avoided asking you which this is up to now (August 2013), because there is no obviously right answer.
© Jim Clark 2011 (modified August 2013) |