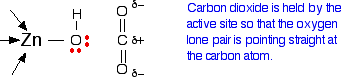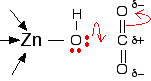|
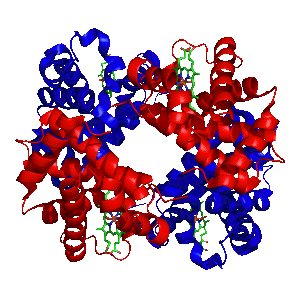
Chemguide: Support for CIE A level Chemistry Learning outcome 11.1(m) This statement looks at several metals that are essential to life, and how they are involved in cell chemistry. Before you go on, you should find and read the statement in your copy of the syllabus. The biochemistry involved in this section can be quite complicated, and it is difficult to judge exactly how much detail you need to know. The syllabus statement suggests that you should be able to explain the chemistry of what is happening if you are given information in the question. But you also need a certain amount of knowledge as well. Up to the November 2010 exam session, nothing really detailed had been asked on any of this. In what follows, there is probably more detail than you really need. At the very least, make sure that you can say where each metal is involved in cell biochemistry, and give a brief sentence (worth probably just 1 mark) about its role. Iron in haemoglobin Haemoglobin is found in red blood cells, and you are obviously aware that it is involved in carrying oxygen around the body. Here is haemoglobin, using the sort of models of proteins that you will have come across before when we looked at enzymes:
| |
|
Note: The diagram comes from this Wikipedia page. | |
|
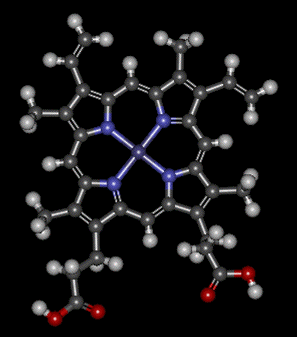
Haemoglobin consists of four protein sub-units joined together. These are of two kinds - shown in the diagram in red and blue to show the difference between them. What are really important, though, are the four smaller groups shown in green - one for each of the protein sub-units. These are haem (US: heme) groups. If you are working through this CIE section in order, you will probably have come across the haem group as an essential part of the enzyme catalase. The same group is used in haemoglobin - the difference being that now the iron is present as iron(II) rather than iron(III). This is a model of haem:
| |
|
Note: The diagram comes from this Wikipedia page. DO NOT learn this structure! | |
|
The iron(II) ion is at the centre of this, and is attached to the blue nitrogen atoms via dative covalent (co-ordinate covalent) bonds. Lone pairs on the nitrogen bond into empty orbitals on the iron. Remember that! It is an important bit of the chemistry of the haem molecule. All these bonds are in the same plane. But iron normally has a co-ordination of six. Six groups can attach around the central iron in an octahedral arrangement. So what is in the other two positions? One of the positions is where it is attached to the protein sub-unit via another dative covalent bond involving a nitrogen on a particular amino acid residue. But it is the other position which is interesting. When haemoglobin isn't carrying oxygen, the sixth position on the haem group is occupied by a loosely-bound water molecule. (Yet another dative covalent bond.) But that water molecule is easily swapped for an oxygen molecule, O2, again via a dative covalent bond. This process is reversible.
(We tend to leave the relatively unimportant water molecules out of these equations. As a chemist that offends me, but we are into biochemistry here!) Now, don't forget that the haemoglobin molecule contains four haem groups, and so the haemoglobin as a whole can attach four oxygen molecules. In other words, a particular molecule of haemoglobin can carry a total of 8 oxygen atoms. When it is carrying all these oxygens, it is known as oxyhaemoglobin.
In blood in contact with oxygen in the lungs, the concentration of oxygen is high, and so the position of this equilibrium lies strongly to the right. Oxygen is picked up readily by the haemoglobin. This travels around the body in the blood. When it gets to wherever it is needed, the concentration of oxygen there will be much smaller, and so the equilibrium will move to left. The haemoglobin stripped of its oxygen then returns to the lungs via the blood system. All of this is essentially just an application of Le Chatelier's Principle. | |
|
Note: Some carbon dioxide is transported back to the lungs via haemoglobin, but it doesn't bind to the haem group. Instead it binds to another site on the protein. On the other hand, carbon monoxide does bind to the haem group - and does so much more strongly that oxygen does. Carbon monoxide is poisonous because it ties up the haemoglobin in a form that the oxygen can't use. | |
|
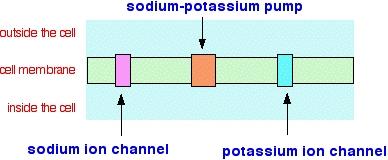
What sort of exam questions are asked about this? In the 13 exam sessions up to June 2013, this has been asked three times. Two questions asked you for the location of iron in the organism, and its role, for a total of two marks. You could have got these by saying that it was present in red blood cells or haemoglobin, and that its role was to transport oxygen around the blood. The other question gave you the structure of haem and some information about haemoglobin, and asked three simple questions about it - how many atoms of oxygen one haemoglobin molecule could carry; what the bonding was holding the iron in place, and what the geometry of the bonding around the iron was. A total of three easy marks. Sodium and potassium ions in the transmission of nerve impulses This is a seriously complicated bit of biology/biochemistry, but the only question asked about it in the first eight exam sessions of the current syllabus was trivial and worth only 2 marks. I will keep it as simple as possible. What we are interested in as far as the CIE syllabus is concerned is the role of sodium and potassium ions in transmitting nerve impulses. There are three different structures in nerve cell membranes that are involved in this:
Each of these depends on protein molecules which lie across the cell membrane - partly inside the cell and partly outside it. And one more thing you need to understand before we begin . . . When the nerve cell is at rest, there is
The various sites that allow movement of sodium or potassium ions into or out of the cell are embedded in the cell membrane.
The sodium and potassium ion channels You can think of these as protein "gates" which can be opened to allow sodium or potassium ions to move into or out of the cell. The direction of movement is the obvious one. Ions will move from where there are lots of them to where there are fewer of them. We say that the ions are moving down their concentration gradient. That means that if the sodium channel is open, sodium ions will move through it into the cell. If the potassium channel is open, potassium ions will move out of the cell. We need to explain why the two ions can't use each other's channel. Why can't sodium and potassium ions use each other's channels? You must realise that we aren't talking about simple diffusion here. The positive ions are moved through the ion channels via attractions set up with the protein molecules lining the walls. That means that the ions must be the right size and be able to form the right bonds with sides of the channels. It isn't just the size of the ions that matters. Potassium ions are bigger than sodium ions, and so you would think that sodium ions would be able to pass through the potassium channel as well as the potassium does. The problem is that the sodium ions are too small for proper effective attractions to be made with both sides of the ion channel, and so they don't get moved through the channel. The hydration of the ions also matters. The positive ions in solution will have water molecules attracted to them. It takes energy to remove these water molecules. The attractions between the larger potassium ions and the water molecules are weaker than they are in the sodium case, and so it is easier to remove them. These water molecules are stripped off the potassium ions before they pass through their channel. The energy to do this is recovered by making new attractions between negatively charged parts of the protein side groups and the positive potassium ions. Once the potassium ions get inside the cell, they are hydrated again. The energetics of all of this is unfavourable for sodium ions. The situation is slightly more complex in the sodium ion channel. Sodium ions have to have a water molecule attached in order to interact properly with the groups lining the sides of the channel. Potassium ions with water molecules attached are the wrong size for this to work. The sodium-potassium pump This is what is responsible for keeping the sodium and potassium ion concentrations different on each side of the cell membrane. It works by pumping sodium ions out of the cell and potassium ions back in. For every 3 sodium ions it removes, it brings 2 potassium ions back in again. The name "pump" implies that energy has to be used in doing this, and that is indeed the case. The source of the energy is ATP. There is a very good simple animation of this process which you can find by following this link. | |
|
Note: Using external links is always a bit of a risk, because pages get moved or deleted. If you have any problems accessing this page, please let me know via the address on the about this site page. | |
|
How is all this involved in the transmission of nerve impulses? The resting potential of the cell When nothing interesting is happening to a nerve cell, there is a potential difference across the membrane between the outside and inside of the cell. This is represented by a small voltage of -70 mV (millivolts). The minus sign shows that the inside of the cell is negative with respect to the outside. That is because the various negative charges on protein side chains or on the phosphate groups of DNA aren't completely covered by the positive ions in the cell solution. Stimulating the cell When the cell gets stimulated, the sodium ion channel immediately opens, and sodium ions flood into the cell. The extra positive charge associated with these causes a rapid change in the potential difference to about +30 mV. This is called the action potential. When this is reached, the sodium channel closes, and the potassium channel opens. The cell loses positive potassium ions, and the potential difference goes negative again. Then the potassium channel closes again. Finally, the original concentrations of sodium and potassium ions in the cell are re-established using the sodium-potassium pump to remove the extra sodium ions from the cell, and replace them with potassium ions. How does this transmit the nerve impulse? Nerve cells have very, very long "tails" called axons. The various channels we have been talking about occur all the way along the axons. The changes in the electrical properties at one part of the axon stimulate the same thing to happen to the next group of channels, which in turn stimulate the next group - and so on down all the whole length of the axon. What sort of exam questions are asked about this? There have been two questions involving this in the 13 exam sessions up to June 2013. One question asked you for the location of sodium in the organism, and its role, for a total of two marks. You could have got these by saying that it was present in nerve cells, and that its role lay in the transmission of nerve impulses. The other involved a similar question about potassium, plus an additional question which partly relied on you recognising that potassium ions were involved in water retention in cells. I have no idea how you are expected to know that. It isn't mentioned by the syllabus (which talks only about transmission of nerve impulses). Neither version of the Support Booklet mentions this, and neither does the Coursebook. A quick Google search finds lots about ion pumps, but only an odd vague reference to fluid balance. An unfair question (June 2012 paper 42 Q6). Zinc ions in carbonic anhydrase This is a modified version of what you will probably have already come across on the Chemguide page about proteins as enzymes. I have cut out as much as possible of what you won't need to know.
Carbonic anhydrase is an enzyme which catalyses the conversion of carbon dioxide into hydrogencarbonate ions (or the reverse) in the cell.
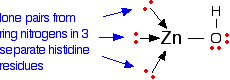
The zinc ion is a cofactor and is bound to the enzyme via dative covalent (co-ordinate covalent) bonds from lone pairs on the nitrogen atoms in histidine residues. Simplifying the structure around the zinc . . .
The arrangement of the four groups around the zinc is approximately tetrahedral. Notice that I have distorted the usual roughly tetrahedral arrangement of electron pairs around the oxygen - that's just to keep the diagram as clear as possible. So that's the structure around the zinc. How does this catalyse the reaction between carbon dioxide and water? A carbon dioxide molecule is held by a nearby part of the active site so that one of the lone pairs on the oxygen is pointing straight at the carbon atom in the middle of the carbon dioxide molecule. Attaching it to the enzyme also increases the existing polarity of the carbon-oxygen bonds.
If you have done any work on organic reaction mechanisms at all, then it is pretty obvious what is going to happen. The lone pair forms a bond with the carbon atom and part of one of the carbon-oxygen bonds breaks and leaves the oxygen atom with a negative charge on it. This is a nucleophilic attack on the slightly positive carbon atom at the centre of the carbon dioxide. (Notice this! The syllabus specifically mentions it.)
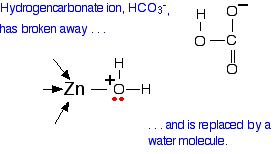
What you now have is a hydrogencarbonate ion attached to the zinc. The next diagram shows this broken away and replaced with a water molecule from the cell solution.
All that now needs to happen to get the catalyst back to where it started is for the water to lose a hydrogen ion. What sort of exam questions are asked about this? In the 13 exam sessions up to June 2013, this has been asked twice. The questions asked you for the location of zinc in the organism, and its role, for either one or two marks. You could have got these by saying that it was present in carbonic anhydrase, and that its role lay in converting carbon dioxide into hydrogencarbonate ions. The syllabus mentions it as an enzyme cofactor - it would make sense to use that term as well.
© Jim Clark 2011 (modified August 2013) |
|