|
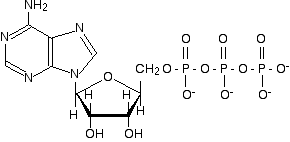
Chemguide: Support for CIE A level Chemistry Learning outcome 11.1(l) This statement is about the function of ATP in the cell as a source of energy. Before you go on, you should find and read the statement in your copy of the syllabus. When you are reading about this, you must remember that you are doing a chemistry exam - not biology or biochemistry. You do NOT need to remember complicated formulae. Block diagrams of the sort that you met in DNA chemistry will be fine. The structure of ATP ATP stands for adenosine triphosphate. It is made from the base (adenine), a sugar (ribose) and three phosphate groups joined up in a row. Here is the full structure:
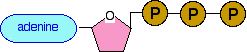
This is a structure remarkably similar to the nucleotides used to build up RNA. (Notice that we have to compare it with RNA rather than DNA because the sugar is ribose, and not deoxyribose.) The only difference is the presence of 3 phosphate groups rather than 1. In the same sort of way we did for the components of DNA and RNA, we can simplify this to a block diagram which is perfectly adequate for what we want to talk about:
Why ATP is so important in the cell ATP is the cell's main source of energy. Energy is needed for all sorts of purposes - for example:
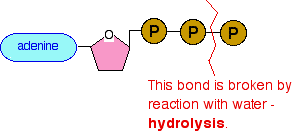
The most obvious of these to remember as a chemistry student is the need for an energy source to make endothermic changes happen. In the lab, we can help things along by heating them with a Bunsen. You can't provide energy in the body in that way. The bonds between the phosphate groups are weakened by repulsions between lone pairs (including full negative charges) on all the oxygens surrounding the phosphorus atoms. If one of the bonds between the phosphate groups is broken, more energetically stable substances are formed, and so energy is released. Most of that energy doesn't get released as heat. Instead, it is passed directly to the reaction which needs it. Using the block structure, what happens to the ATP is this:
The last phosphate group breaks off to produce what is usually just described as "inorganic phosphate", and given the symbol Pi. The rest of the structure with two phosphate groups remaining is called adenosine diphosphate - ADP. This change releases about 30 kJ mol-1 of energy. Here is the simplified equation normally used. This is the version given by the CIE syllabus.
There is quite a high activation energy for this reaction, and it only happens in the presence of suitable enzymes. ATP is a good example of a coenzyme - an essential part of the enzyme's ability to catalyse a reaction between other things. Let's assume that you have a simple reaction between two substrates (reactants), and that their reaction is endothermic. In order for a reaction to happen, the ATP has to attach to the enzyme's active site alongside the substrates. The energy needed to carry out the reaction involving the substrates is generated by breaking the phosphate-phosphate bond in the ATP. That energy is then passed directly to the substrates to make their reaction happen. How is ATP regenerated? Since ATP is converted to ADP in any number of cell reactions, it is obvious that there has to be some other process to regenerate it. If energy is released when ATP converts into ADP, it is also obvious that energy is going to have to be found from somewhere to convert the ADP back to ATP again. Carbohydrates or fats that you eat are eventually converted into carbon dioxide and water by lots of small steps in the cells of your body. As this happens, large amounts of energy are released. For example, the enthalpy change of combustion of sucrose (ordinary sugar) is -5644 kJ mol-1. That means that when 1 mole of sucrose is burnt in oxygen to give carbon dioxide and water, 5644 kJ is released. Although obviously sucrose doesn't burn in the body, exactly the same amount of energy is released when 1 mole of sucrose is converted into carbon dioxide and water in the presence of oxygen using cell processes. Some of that energy is used to convert ADP and inorganic phosphate back into ATP again. This mainly takes place in parts of the cell known as mitochondria and, if you like to collect long words, it is known as oxidative phosphorylation. But remember that this isn't a biology or biochemistry exam that you are preparing for. For the 13 exam sessions up to June 2013, questions about ATP were only asked twice, once for 3 marks, and once for 2 marks. All that was wanted was
| |
|
Note: I haven't mentioned proteins in all this. In fact, protein metabolism also releases energy, and this can also be used to remake the ATP. The reason for leaving it out is that it is more complicated for a chemist to relate to, because you have to worry about what happens to the nitrogen. In any case, most of the energy needs of the body come from carbohyhdrates and fats. | |
© Jim Clark 2011 (modified August 2013) |
|