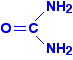|
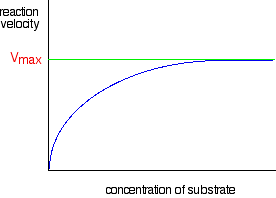
Chemguide: Support for CIE A level Chemistry Learning outcomes 11.1(d) and 11.1(e) These statements are mainly about enzymes. Before you go on, you should find and read the statements in your copy of the syllabus. Main reading You should read the three pages about enzymes, starting with this one. You will find a link to the next page in the series at the bottom of each page you read. Throughout these pages, you will find some detailed descriptions of particular enzyme reactions used as examples. It is important that you just see them as examples to give you an understanding of how enzymes work. They are NOT there for you to learn. If you read these pages carefully, you will have a good general understanding of how enzymes work, and the factors affecting them. We will look at some of the detail that CIE want below. Don't go on to that, though, until you are reasonably confident that you understand what you have read on the Chemguide pages. Extra material for CIE exam purposes Curves for reaction rates against substrate concentrations This graph comes from the second page about enzymes that you have just read.
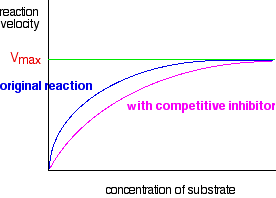
The graph levels off at a maximum reaction rate of Vmax. This happens when all the enzyme molecules are working as fast as they can. Once that happens, increasing the concentration of the substrate can't make the reaction go any faster. CIE have asked two questions (up to November 2010) relating this graph to what happens in the presence of either a competitive or a non-competitive inhibitor. If you aren't reasonably happy about the types of inhibitors, and how they affect enzymes, read the last page about enzymes before you go on. You won't understand the next bit unless you do that (if you need to). What happens to the graph in the presence of a competitive inhibitor? At a relatively low substrate concentration, the rate of the reaction will be less in the presence of a competitive inhibitor. The competitive inhibitor is just getting in the way of the substrate by attaching itself to some of the active sites. However, as the substrate concentration increases, the substrate out-competes the inhibitor. If there is a lot more substrate than inhibitor, the chances are far higher that a substrate molecule will hit an active site than an inhibitor molecule will. That means that at a high enough substrate concentration, the maximum reaction rate will again be Vmax.
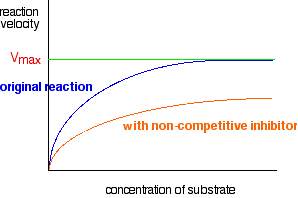
What happens to the graph in the presence of a non-competitive inhibitor? This is quite different. Once a non-competitive inhibitor has attached itself to the enzyme, that particular enzyme molecule won't work any more. The attachment may be reversible, but even if it is, there will be a proportion of the enzyme molecules which are out of action at any one time. That means that however much you may increase the concentration of the substrate, you will never reach the original Vmax. So a graph involving a non-competitive inhibitor looks like this:
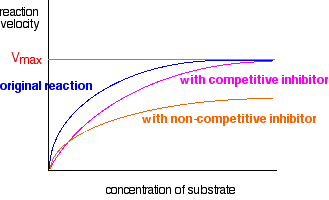
There is now a new Vmax, lower than the one where there was no inhibitor present. Combining these two graphs In November 2008 paper 4 question 8, CIE asked a question which wanted you to put both of these curves onto the same axes. The mark scheme showed what they expected.
You might think that is pretty straightforward, but it isn't. In order to get the mark for the non-competitive line, it had to start off more steeply than the other one, and then cross it - as I have shown in this diagram. The Examiner's Report said that candidates' curves rarely did that. Update December 2012 I spent quite a long time trying to discover whether such curves always crossed, and if so, why they crossed - but failed completely. Then, about a month ago, it was pointed out to me that a version of these graphs was included in the Revision Guide for CIE A level Chemistry by David Bevan and Mary Jones, but in that version, the curves do not cross. Since that book is endorsed by CIE, and contradicted the November 2008 published mark scheme, I emailed CIE to find out exactly what they now want students to do about this. They are currently saying that the either of these inhibitor curves could be the steeper one initially. In other words, the curves may or may not cross depending on the conditions. In their words: "The initial rate of a non-competitively inhibited reaction could be greater or less than that of a competitively inhibited reaction. If greater, the curves will cross; if less, they won't cross. If an identical question was set in the future then this would be the appropriate response." Since the non-crossing version is given in the Revision Guide, in the future it would be perverse of the CIE examiners to demand a version in which they crossed. They would lay themselves wide open to challenge. I don't think I would worry too much about this from now on. More about statement 11.1(e) This statement is about the interaction of proteins with small molecules (or ions). You will already have read about this in relation to enzyme cofactors and inhibitors. But the statement also mentions the ability of small molecules (or ions) to disrupt protein-protein interactions or to block ion channels. You will find out more about ion channels in statement 11.1(m). In truth, as a non-biologist, I know nothing about protein-protein interactions in the cell, except that there are lots of cases where it is important for proteins to bind to each other. The fact that I don't know any detail about this doesn't worry me at all. All I need to know is that the attractions between two different protein molecules are likely to be exactly the same as those which hold a single protein molecule in its tertiary structure. If you read the first bit of the statement, it says "given information, use core chemistry to explain how small molecules interact with proteins . . .". That means that a question will give you the necessary information about the biochemistry, and then ask you how or why a particular molecule or ion can disrupt the attractions between protein molecules or within a protein molecule. You should be able to work that out from what you already know. For example, urea can chemically denature proteins. Urea is:
Denaturing means that the tertiary structure is broken down. So, what part of the bonding that contributes to tertiary structure might urea interfere with? You have sulphur-sulphur bridges, ionic interactions, hydrogen bonds and van der Waals dispersion forces. Which of these has any relevance to urea? Not sulphur-sulphur bridges - these are broken by heavy metals like silver or mercury ions. Urea isn't ionic and so isn't likely to affect ionic interactions. But urea has lone pairs on electronegative elements, and also hydrogen atoms attached to some of those elements, and so will form hydrogen bonds. You can therefore work out that urea is likely to interfere with hydrogen bonds in the tertiary structure of a protein. So far (up to June 2013), any questions in this area have involved either silver ions or mercury ions. In the mercury cases, it has involved breaking sulphur-sulphur bridges. In the silver ion case, you were told that the ion reacted with a -COOH group in the side chains of the amino acid residues, and asked to suggest what types of R group interactions that would affect. The reaction was:
You were told that the silver salt formed was partially covalent. The -COOH group will be involved in the formation of hydrogen bonds and of ionic interactions. The ionic interactions will happen if a hydrogen ion is transferred to a nearby -NH2 group. If the -COOH group has been turned into a partially covalent -COOAg group with a strong bond from the silver to the rest of the group, neither of these can happen. This isn't something you need to learn, because there are endless possible questions that the examiners could think up. You have to have enough confidence in your understanding of the topic to work these things out from what you already know and understand.
© Jim Clark 2011 (last modified August 2013) |