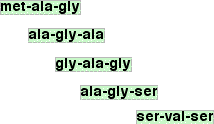|
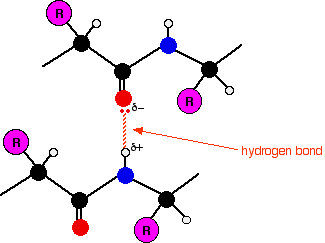
Chemguide: Support for CIE A level Chemistry Learning outcomes 11.1(a), 11.1(b) and 11.1(c) These statements are about the structure of proteins. I am including them all together because you will find all the information you need on a single Chemguide page. Before you go on, you should find and read the statements in your copy of the syllabus. Introduction Unless you have been learning about amino acids recently, before you do anything else, follow this link and read the first section headed "What are amino acids?" You don't need to go any further than this for the present topic. Main page to read Now read the page about the structure of proteins. In common with most people, CIE count sulphur bridges as a part of the tertiary structure of the protein. Points to notice for CIE exam purposes A relatively common question asks you for diagrams (or an explanation) showing the types of bonds which contribute to the primary, secondary and tertiary structures. This is easy stuff, and you can't afford to waste marks by getting it wrong. For primary structures You should realise that the bonds which make up the primary structure are all covalent, and you should be confident that you can draw a short length of protein (or other polypeptide chain), showing the exact bonding in the peptide link. For secondary structures You should know that secondary structures are held together with hydrogen bonds involving the atoms in the peptide links. A lone pair on the double bond oxygen is attracted to the hydrogen attached to the nitrogen atom in a second peptide link in a different part of the chain. If you aren't clear about this, go back and read the protein structure page again. Repeating the essential diagram from that page:
Both alpha-helices and beta-pleated sheets result from hydrogen bonds between these two groups. But it is important to realise that the detailed arrangements are different in the two structures. So how might this be asked in an exam? One CIE question gave you the structure of a bit of protein chain in a beta-pleated sheet, and asked you to draw the bit of chain that this was attached to, showing the bonds that are formed between the two. As long as you understand what a beta-pleated sheet is, that isn't too difficult. In another question, you were asked to name the two forms of secondary structure for a mark each. That's easy, of course. The question then went on to ask you to draw a diagram to show one of these, and that was worth another 2 marks. The mark scheme and examiner's report weren't clear about how much of the structure you were expected to show. The marks went for:
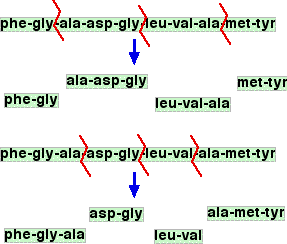
Based on the marks available, you have only got about 2 minutes to do this. I would suggest you keep it simple. For the helix, draw a helix shape as shown in green on the page you have just read. Draw just enough of the chain on the top two coils to show perhaps two of the hydrogen bonds. For the beta-pleated sheet, draw the pleated sheet as shown in green on that page. Then on just two of the strands, draw enough of the chain to be able again to show a minimum of two of the hydrogen bonds. If you spend a bit of time working out how to do this, it should help you to learn how both of these structures fit together. For tertiary structures The tertiary structure involves interaction between side groups on the protein chain - NOT between atoms in the peptide links. You must know the four ways in which the tertiary structure is held together. They are: Sulphur-sulphur bridges These are simple covalent bonds formed between two cysteine residues. Cysteine has a side group containing the -SH group. These react together with the loss of the hydrogen (this is an example of oxidation - CIE asked this once). Sulphur-sulphur bridges consist of single bonds holding two bits of the chain together via the link -S-S-. Try to tie together the words cysteine and sulphur-sulphur bridge in your mind, so that whichever one you see, you immediately think of the other one. Ionic interactions These come from ionic groups on side chains - between -NH3+ in one side group and -COO- in another one. Look for the presence of -NH2 and -COOH groups in side chains. If they get close together, there will be a transfer of a hydrogen ion from one to the other to give these ionic groups. Hydrogen bonds Take care not to confuse these with the hydrogen bonds that hold secondary structures together - those involve atoms in the peptide links. What we are talking about now are hydrogen bonds involving the side groups. This will involve typical hydrogen bonding groups like -OH or -COOH or -NH2 groups. (But don't forget that -COOH or -NH2 groups close to each other will transfer a hydrogen ion, and give you ionic attractions rather than a hydrogen bond.) Van der Waals dispersion forces These are most important where you have reasonably big hydrocarbon groups in the side chains - but they will be there to some extent in the interactions between every side group. Questions about working out amino acid sequences in polypeptides It isn't at all clear from the syllabus or the Chemistry Applications Support Booklet that questions of this sort would be asked. But in the papers that I had available at the time of writing (up to June 2013), this had been asked twice. The questions involved taking a shortish polypeptide and breaking it up into random fragments, and then asking you to work out what the polypeptide was from the fragments. This is easier to explain if we first have a look at the fragmentation process. Fragmenting a polypeptide Suppose you had this polypeptide:
It is possible to split this randomly into fragments by hydrolysing the peptide links in a controlled way so that not all of them get broken. The diagram shows this random splitting happening to two molecules of a polypeptide.
This would be happening over huge numbers of molecules of the polypeptide, and so you would end up with a mixture of fragments from individual amino acids up to perhaps some unbroken original molecules. What CIE have done in their questions is select a collection of tripeptides - fragments containing exactly three amino acids residues. They have then asked you to work out what the shortest possible structure of the original polypeptide might be. Working back to the original sequence This isn't very difficult, but you have to take your time over it and make sure that the answer you come up with accounts for all the fragments. Suppose you were told that partial hydrolysis of a polypeptide gave the following tripeptide fragments:
You are told that the N-terminal amino acid is methionine (met). Conventionally, the N-terminal is the left-hand amino acid residue. That's true of the original polypeptide and also each of the fragments. You have to come up with the shortest possible chain which could produce these fragments. So where do you start? Well, you know that methionine (met) must be at the left-hand end of the chain, and luckily there is only one of those in any of the fragments. So start with that.
What could come next? To try to get the shortest possible chain, you need as much overlap between the various fragments as possible. One possible overlap would be this:
That would come from an original chain met-ala-gly-ser being broken at two different places. But there is another possibility with the same degree of overlap:
How do you know which is right? You would have to think about what happens next along the chain. Suppose the first of these possibilities was right. Here it is again:
What would come next after that? There is only one other fragment with serine (ser) in it. That would have to overlap just the serine on the end.
But there is no way of continuing after that. You haven't got any remaining fragments which contain serine. So this is a dead-end. We'll have to explore the other possibility. Here it is again:
There is an obvious double overlap you can do on this:
And the rest is equally obvious:
Notice that it was only possible to get a single overlap on the last fragment. That's fine. If that is all that is possible, you have no choice. In fact there have been other points during this where a single overlap was possible. I have avoided pointing them out because they would lead to a longer chain, probably wouldn't have worked anyway, and would just complicate everything even more. In situations like this, look for double overlaps if you can find them. Only use single overlaps if all else fails. So what was the original polypeptide?
Make sure that you can see exactly how that derives from the last diagram. When you have finished all this, make sure that you have used up all your fragments. It isn't difficult to forget one when they are just printed in a single line in an exam paper. All of this bother is going to be worth, probably, 2 marks! You need to practise as much as possible. Try making up your own examples with friends.
© Jim Clark 2011 (last modified August 2013) |