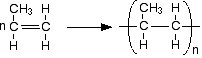|
Chemguide: Support for CIE A level Chemistry Learning outcomes 10.8(a) and 10.8(b) These statements are about addition polymerisation. Before you go on, you should find and read the statements in your copy of the syllabus. Statement 10.8(a) General If you are working through the syllabus, you will already have come across this as a part of statement 10.2(d)(iv). I suggest you start by re-reading my CIE statement 10.2(d). You just need to revisit part (iv) of this statement towards the bottom of the page. I don't really need to add anything to the advice given on that page. The current statement asks for poly(ethene) and PVC, so make sure that you really understand how their monomers join to make the polymer. But don't restrict yourself to these examples. CIE could ask you about the relationship between any monomer and its addition polymer, however unfamiliar. The best way of working out what you need to be able to do is to look through as many past papers as you can find. Drawing the structures of polymers Pay particular attention to the way that this is done in simple equations on the page about polymerisation of alkenes. For example:
For exam purposes, when you are drawing these:
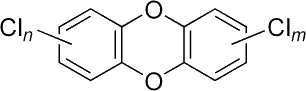
Statement 10.8(b) This is about problems with the disposal of poly(alkenes). The syllabus mentions two problems. The first is that poly(alkenes) are non-biodegradable. In other words they aren't broken down in the environment, or else are only broken down very slowly. The teacher support material mentions that the problem is worse for branched chain poly(alkenes) - those with hydrocarbon side groups. The other problem concerns the disposal of chlorinated polymers like PVC. If these are burnt at too low a temperature, poisonous products such as phosgene (COCl2) and dioxins are formed. Phosgene was a First World War poisonous gas, and dioxins are extremely toxic. For interest only, dioxins are a family of related compounds with varying numbers of chlorines. The "m" and "n" in the diagram (taken from Wikipedia) can range from 0 to 4, and can be attached in various positions on the left and right-hand rings.
© Jim Clark 2011 (modified August 2013) |