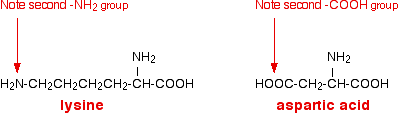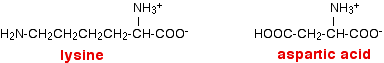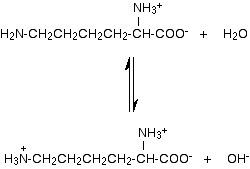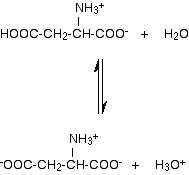|
Chemguide: Support for CIE A level Chemistry Learning outcome 10.7(i) This statement is about amino acids. Before you go on, you should find and read the statement in your copy of the syllabus. This statement will only be examined in the final exam of a two year course. Important background Read the page introducing amino acids. You should read down as far as the optical activity of the amino acids (as a useful revision), but you do not need the last bit about D and L configuration. The acid-base behaviour of amino acids Simple amino acids I am taking these to be amino acids where the R group doesn't contain either an amino group or a carboxylic acid group. Read the page about the acid-base behaviour of amino acids. You can ignore the last section titled "Why isn't the isoelectric point of an amino acid at pH 7?" Amino acids with other amino or carboxylic acid groups present These include amino acids like lysine or aspartic acid.
Let's assume that only the groups at the right-hand end of these molecules become involved in forming zwitterions. The zwitterions would be:
In solution in water, the other groups would react with the water. For example, the basic amino group at the left-hand end of the lysine molecule would accept a hydrogen ion from the water like this:
Notice that the new ion has now got 2 positive and 1 negative charge, giving a net charge of +1. In fact it didn't matter which of the two amino groups we assumed was a part of the original zwitterion - you would end up with the same final ion. | |
|
Note: This is tied in with the Applications part of the syllabus as well as this statement. The Applications Support Booklet said that an amino acid like lysine will have a +1 charge at pH 7, but doesn't explain this properly. It shows the reaction above as a one way process rather than an equilibrium. The reaction immediately above is an equilibrium, but the position of equilibrium is well towards the original zwitterion and water. That means that if you add lysine to water, only some of the amino acid will exist as an overall 1+ ion; most will still be the original zwitterion. Because of the hydroxide ions formed, the solution will be alkaline - not pH 7. Suppose, though, that you held the pH at exactly 7. In pure water, the solution will be slightly alkaline due to the hydroxide ions produced. To force the pH down to 7, you would have to remove these by adding acid. If you remove them, the equilibrium will move to replace them, and more and more of the overall 1+ ion will be formed, until virtually all of the lysine will be there in that form. | |
|
In aspartic acid, the acidic -COOH group at the other end also reacts with water, losing a hydrogen ion to a water molecule.
Notice that this time, the final ion has a net 1- charge, and a solution of aspartic acid in water would be acidic. | |
|
Note: Again, the Applications Support Booklet doesn't explain properly why the aspartic acid would carry an overall 1- charge if you fixed the pH at 7. This time, the solution formed in water would be acidic because of the hydroxonium ions formed. But again, suppose that you held the pH at exactly 7. To force the pH up to 7, you would have to remove the hydroxonium ions by adding an alkali. If you remove them, the equilibrium will move to replace them, and more and more of the overall 1- ion will be formed, until virtually all of the aspartic acid will be there in that form. | |
© Jim Clark 2011 (modified August 2013) |
|