|
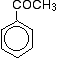
Chemguide: Support for CIE A level Chemistry Learning outcome 10.5(a) This statement is about the formation of aldehydes and ketones by the oxidation of alcohols, their reduction back to alcohols using NaBH4 or LiAlH4, and their reactions with HCN and NaCN. Before you go on, you should find and read the statement in your copy of the syllabus. Background work Start by reading the page introducing aldehydes and ketones. This covers naming and physical properties, together with a brief introduction to the reactivity of the compounds. Throughout the Chemguide pages in this section, you will find that I have deliberately left out aldehydes or ketones containing a benzene ring. CIE do, however, want you to know about phenylethanone, which is a ketone made up of a benzene ring with a -COCH3 group attached:
In almost all cases, the presence of the benzene ring doesn't change its characteristic ketone properties. There is one case (which probably won't be asked in an exam) where there is a difference, and I will point that out when it is relevant. Statement 10.5(a)(i): The formation of aldehydes and ketones from alcohols You will find this on the page about making aldehydes and ketones. CIE is happy for you to use the simple versions of the equations with oxygen in square brackets. Statement 10.5(a)(ii): The reduction of aldehydes and ketones You should read the page about reduction of aldehydes and ketones. This contains more information than you need. Concentrate mostly on the first section headed "Background to the reactions".
Other important points about the NaBH4 reaction There are two things which came out of a careful look through past papers. One of these comes up quite commonly, and one was an odd multiple choice question. The common question NaBH4 is a reagent which reduces C=O double bonds, but will not reduce C=C double bonds. The normal reducing agent for C=C double bonds is hydrogen in the presence of a nickel catalyst. Don't be surprised to be given a complicated molecule containing both a C=C double bond and a C=O double bond, and to be asked what the effect would be of using either nickel and hydrogen, or NaBH4. You have to remember that nickel and hydrogen reduces C=C double bonds; NaBH4 reduces C=O double bonds. The odd multiple choice question In the Summer 2008 multiple choice paper (paper 1 Q 28), CIE asked a question about the mechanism for this reaction. That isn't specifically on the syllabus. It told you that ethanal can be reduced using NaBH4, and that the reaction was nucleophilic addition, and asked you to choose a reaction which could be the first step of the mechanism. The choices involved attack by either H+ or H- on either the carbon or the oxygen atom in the carbonyl group. The only way you could work this out is by deduction from the fact that you are told that it is nucleophilic addition. In other words, the attack is by a nucleophile - something with a negative charge or, at least, an active lone pair of electrons. Attack must therefore be by a hydride ion, H-, on something which has got some degree of positive charge - the carbon atom in the carbonyl group. If CIE ask these off-the-syllabus questions, usually (but not always!) you can work out the answer provided you understand the surrounding chemistry. | |
|
Note: I'm not happy with this question, not because you have to think about it, but because the chemistry is wrong! The initial attack doesn't involve a hydride ion - it involves the whole of the BH4- ion.
I know that the examiners could argue their way out of this, because the question is phrased "What could (my italics) be the first step of the mechanism?". But it still leaves a misleading impression. | |
|
The LiAlH4 reaction At the time of writing, the LiAlH4 reaction had only just been added to the syllabus, and nothing specific had been asked in the AS exam in 2013. It is hard to judge what CIE might ask about it. Make sure that you can write the equation in terms of hydrogen in square brackets (exactly the same as the NaBH4 equation), and that you know the conditions for the reaction. In particular, be aware that you can't use LiAlH4 in the presence of water or alcohols. My best guess is that, apart from the conditions, the questions will be much the same as for NaBH4. Statement 10.5(a)(iii): The reaction of aldehydes and ketones with HCN and NaCN You should read the first part of the page about simple addition to aldehydes and ketones. You don't need the reaction with sodium hydrogensulphite. In CIE questions they tend to talk about using hydrogen cyanide in the presence of some cyanide ions (from sodium or potassium cyanide). You will understand the reason for this when you look at the mechanism in the next statement 10.5(b). Don't worry about the reactions mentioned in "Uses of the reaction" for now, although it might be a good idea to come back and have a look at it when you have finished all the organic chemistry. It wouldn't be too difficult to make up an exam question out of this.
© Jim Clark 2011 (last modified August 2013) |
|