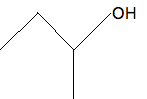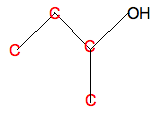|
Chemguide: Support for CIE A level Chemistry Learning outcome 10.1(k) This statement is about working out the molecular formula of an organic compound from its structure. Before you go on, you should find and read the statement in your copy of the syllabus. If you are given the structural formula of, say, ethanol as CH3CH2OH, it is easy to add up the various atoms to give the molecular formula of C2H6O. If you are given it as a displayed formula, you just have to be careful to count all the atoms. It isn't difficult - you just have to be careful. It is definitely more difficult to do this from a skeletal formula, though. If I was doing this in an exam, I would write in all the missing atoms on top of the structure on the question paper. So, starting with a skeletal formula:
Write in all the carbon atoms:
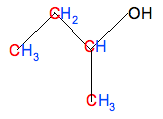
Don't forget that there isn't a carbon atom at the end of a line with something already attached - in this case, an -OH group. And then add enough hydrogens so that each carbon is making four bonds:
Giving the molecular formula C4H10O. Be careful, though, and take your time. It is very easy to miss something - for example, in this case, forgetting to count the hydrogen in the -OH group!
© Jim Clark 2012 (modified August 2013) |