|
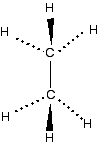
Chemguide: Support for CIE A level Chemistry Learning outcomes 10.1(c) and 10(d) These statements cover the bonding and shapes of ethane, ethene and benzene. It makes sense to take them together because the shapes are dependent on the bonding. Statement 10(c) also wants you to be able to predict the shapes of similar molecules. As long as you understand the bonding in ethane, ethene and benzene, that is quite easy to do. Before you go on, you should find and read the statements in your copy of the syllabus. Notice that benzene is in bold type in the syllabus statements. That means that it won't be examined until the final exam in a two year course. Before you start, you should revise electronic structure and atomic orbitals by reading this page in the Basic Organic Chemistry part of Chemguide. Bonding and shapes of methane and ethane I know that methane isn't specifically mentioned by the syllabus, but you can't understand ethane without first understanding the bonding in methane. You should read the page bonding in methane which includes both methane and ethane. Don't skip over the methane bit and go straight to ethane. It won't make any sense unless you read the whole page. If you are asked to draw the shape of an ethane molecule, I suggest you use something like this:
| |
|
Note: If you have forgotten what the various ways of drawing bonds mean, re-read the page on how to draw organic molecules. | |
|
The bonding and shape of ethene You will find everything you need on the page bonding in ethene. By the end of this, you should understand that:
The bonding and shape of benzene You should read this only if you are in the last year of a two year course. It assumes that you have met quite a lot of chemistry already. Chemguide has two pages about the structure of benzene. The first discusses the older Kekulé structure for benzene, and you should read this because you will still find this structure in use, and you need to know what it means. It is also important to understand that benzene is much more energetically stable than the Kekulé structure suggests. You need to know about delocalisation energy, which is introduced on this page. You will find what you need in terms of modern bonding theory on the page about the bonding in benzene. You need to understand that:
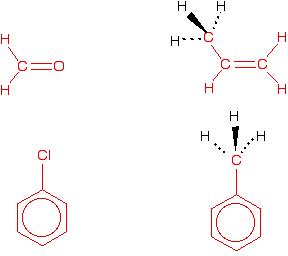
Predicting shapes of similar molecules It is hard to know exactly what CIE want for this, and at the time of writing I couldn't find a question asked about it. As a general guide, remember that:
Here are a few simple examples. The parts of the molecules in red are all planar. The two CH3 groups have a tetrahedral arrangement around the carbon atom.
© Jim Clark 2010 (last modified August 2013) |
|