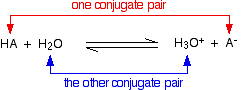|
Chemguide: Support for CIE A level Chemistry Learning outcome 25: Equilibria 25.1: Acids and bases Learning outcomes 25.1.1 and 25.1.2 These statements are about the terms conjugate acid and conjugate base. Before you go on, you should find and read the statements in your copy of the syllabus. You will find this on the page about theories of acids and bases. It would be a good idea to revise the Bronsted-Lowry theory. The section about conjugate pairs follows that. You can ignore the long green box at the end of this, and the rest of the page. The previous syllabus added a comment about the "use of the acid-I base-I, acid-II base-II concept". This is another way of talking about conjugate pairs, which are explained on the page you are about to look at. This isn't mentioned on the current syllabus, but I am repeating what I said previously just in case. When you get to the section on conjugate pairs, you will find this equation:
Using the acid-I, base-II terminology:
If you wrote the reverse form of the equation, then presumably you would call H3O+ acid-I, and A- base-II. HA would then be acid-II, and H2O base-I To be honest, I have spent the whole of my adult life in chemistry education, and had never come across conjugate pairs being described in this way before. It doesn't seem to add much apart from some confusion!
© Jim Clark 2020 |