|
Chemguide: Support for CIE A level Chemistry Learning outcomes 12.1.3, 12.1.4 and 12.1.5 Nitrogen and sulfur Learning outcome 12.1.3 This is about the formation of oxides of nitrogen, and their removal using catalytic converters. Nitrogen monoxide, NO, is formed when an electrical spark is passed through a mixture of nitrogen and oxygen. This happens in the atmosphere during lightning storms. In a petrol (gasoline) engine, a spark is passed through a mixture of petrol vapour and air. The point of doing that, of course, is to ignite the petrol. But it also causes some of the oxygen and nitrogen in the air to combine:
If this escapes into the atmosphere, it causes problems (see below), but it can be removed using a catalytic converter. A catalytic converter uses expensive metals like platinum coated on to a honeycomb structure to give a high surface area. The platinum catalyses various reactions which help to get rid of pollutants from the exhaust gases. In this case, it converts harmful carbon monoxide and nitrogen monoxide into carbon dioxide and nitrogen.
Statement 12.1.4 and 12.1.5 These statements are about why gases such as nitrogen monoxide or nitrogen dioxide are pollutants. The most obvious problem is their role in the formation of acid rain. Nitrogen monoxide reacts with oxygen in the atmosphere to form nitrogen dioxide.
| |
|
Note: Normally you would write this equation without the half in it, by multiplying everything by two. In this case, I am interested in what happens to a single molecule of nitrogen dioxide (see below). | |
|
Nitrogen dioxide reacts with oxygen and water in the atmosphere to produce very dilute solutions of nitric acid - falling as acid rain. But the main cause of acid rain is sulfur dioxide. It is oxidised to give sulfur trioxide which reacts with rain water to give very dilute sulfuric acid.
Nitrogen dioxide acts as a catalyst in the conversion of sulfur dioxide into sulfur trioxide. The nitrogen dioxide first oxidises sulfur dioxide to sulfur trioxide. In the process, the nitrogen dioxide is reduced to nitrogen monoxide.
Now the nitrogen monoxide is converted back to nitrogen dioxide again by reaction with oxygen.
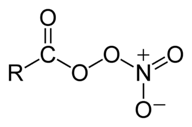
So, the nitrogen dioxide is regenerated at the end of the reaction, and can go on to do the same thing again, and again . . . Nitrogen oxides also contributes to the formation of photochemical smog. They have a role in the formation of ozone, and a pollutant called peroxyacetylnitrate (PAN). This is one of a number of similar compounds called peroxyacylnitrates. The general structure of these (taken from Wikipedia) is
The R group in peroxyacetylnitrate is CH3, but other hydrocarbon chains are possible as well. | |
|
Note: An acyl group is hydrocarbon group attached to a C=O double bond. That is the RC=O group in the formula above. An acetyl group is the old name for a particular acyl group - CH3C=O. It is now properly known as an ethanoyl group. The peroxy part of the name is describing the O-O bond in the structure. One of those oxygens is also, of course, a part of the nitrate group, NO3. Their are various ways of showing the structure of the nitrate group and there is no totally simple way of doing it. I have described another way you can show this at the bottom of the page on co-ordinate covalent bonding, and have discussed the problem at slightly more length. | |
|
Peroxyacetylnitrate (and other peroxyacylnitrates) in smog is dangerous to health, and so is ozone. These are formed by complex reactions involving nitrogen dioxide, unburnt hydrocarbons present in exhaust gases, and oxygen from the air. There is no suggestion in the syllabus that you should know the chemistry of either ozone or PAN formation.
© Jim Clark 2020 |
|