|
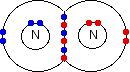
Chemguide: Support for CIE A level Chemistry Learning outcome 12.1 Nitrogen and sulfur Learning outcome 12.1.1 This statement is about the reasons for the lack of reactivity of nitrogen gas. The reactivity of nitrogen is relatively low because of its bonding. The nitrogen molecule, N2, has a triple bond between the two nitrogen atoms. In a simple dots-and-crosses diagram:
This is a very strong bond with a bond energy of +944 kJ mol-1. When nitrogen reacts with other things, it tends to split this bond completely. For example, if nitrogen and oxygen are sparked together, nitrogen monoxide is formed. The nitrogen atoms end up in two different molecules.
Or, magnesium nitride is formed when magnesium is heated in nitrogen. Magnesium nitride is ionic, and this time, you end up with 2 separate nitride ions.
Notice that despite the high bond energy of the nitrogen-nitrogen bond, the last reaction is quite strongly exothermic. Lots of energy is released when the very strong ionic bonds between the 2+ magnesium ions and the 3- nitride ions are set up. The magnesium actually burns in the nitrogen. So it isn't necessarily the case that nitrogen won't react because of its high bond energy. However, the activation energy is going to be high, because of the need to break the very strong nitrogen-nitrogen bond. Other substances with equally high bond energies, such as the triple bond in carbon monoxide, can be more reactive. In the carbon monoxide case, for example, its reactions often produce carbon dioxide. In this case, you aren't having to break the whole of the triple bond. In carbon dioxide, there is still a double bond between the carbon and oxygen. Molecules like carbon monoxide are also polar. Nitrogen molecules have no permanent polarity, and aren't easily polarisable either. Therefore, they aren't so attractive to nucleophiles or electrophiles. | |
|
Note: You will find a simple diagram of the carbon monoxide molecule (just like the nitrogen one above) towards the bottom of the page about dative covalent bonding. If you haven't come across the terms nucleophiles or electrophiles yet, don't worry too much about it for now. An electrophile is attracted to areas of negativeness in other substances; a nucleophile is attracted to areas of positiveness. | |
© Jim Clark 2020 |
|