|
Chemguide: Support for CIE A level Chemistry Learning outcome 11.4 Group 17 (previously Group 7) The reactions of chlorine Learning outcome 11.4.1 This statement is about the reaction of chlorine with sodium hydroxide solution under two different sets of conditions Before you go on, you should find and read the statement in your copy of the syllabus. The reaction with cold sodium hydroxide solution The reaction between chlorine and cold dilute sodium hydroxide solution is:
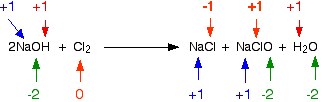
NaClO (sometimes written as NaOCl) is sodium chlorate(I). The old name for this is sodium hypochlorite - and the solution on the right-hand side of the equation is what is normally sold as bleach. Now, think about this in terms of oxidation states. Obviously the chlorine has changed oxidation state because it has ended up in compounds starting from the original element. Checking all the oxidation states shows:
The chlorine is the only thing to have changed oxidation state. Has it been oxidised or reduced? Yes! Both! One atom has been reduced because its oxidation state has fallen. The other has been oxidised. This is a good example of a disproportionation reaction. A disproportionation reaction is one in which a single substance is both oxidised and reduced. The reaction with hot sodium hydroxide solution The reaction between chlorine and hot concentrated sodium hydroxide solution is:
The unfamiliar product this time is sodium chlorate(V) - NaClO3. As before, check the oxidation states of everything in the equation. Once again, you will find that the only thing to have changed is the chlorine. It goes from 0 in the chlorine molecules on the left-hand side to -1 (in the NaCl) and +5 (in the NaClO3). This is also a disproportionation reaction. Building equations for these reactions Actually, the first one is simple, and most people would just write it down. The second one is more difficult, and the teacher support material suggests that you build it up using oxidation states. You would need to have learnt the two main products of the reaction. So write those down:
Now think about the oxidation state changes. To go to NaCl, the oxidation state of the chlorine has fallen from 0 to -1. To go to NaClO3, it has increased from 0 to +5. The positive and negative oxidation state changes must cancel out, so for every NaClO3 formed, there must be 5 NaCl. Write that down:
Now it is a simple job to balance the sodiums and the chlorines. When you have finished, you will find that you have enough hydrogens and oxygens left over to make 3H2O. That seems reasonable!
© Jim Clark 2020 |