|
Chemguide: Support for CIE A level Chemistry Learning outcome 9.1 The Periodic Table Periodicity of physical properties of the elements in Period 3 These statements are about the way various physical properties of the elements vary across Period 3 of the Periodic Table. It is important that you note exactly what CIE want from their syllabus. Statements 9.1.1 and 9.1.2 It doesn't make sense to separate the explanations from the descriptions. Atomic radius You will already have met this in statement 1.1.7 which includes the explanation for the trend. I assume that is why you aren't being asked to explain the atomic radius trend here. You do need to know it! Start by reading the page atomic and ionic radii as far as (but not including) the trends in the transition elements. You won't need that until much later in the course. Notice that the trend in Period 3 is just the same as the one in Period 2, although of course the Period 3 elements are all bigger. This is what is meant in the syllabus by "periodicity". The same pattern is repeating itself. Then find the atomic radius section on the page atomic and physical properties of period 3 elements for a brief summary of what you need to know for these statements. You will be referred back to this page several times later for other things. Ionic radius Again this has been covered in statement 1.1.7 which includes the explanation for the trend - which you do need. Now is a good time to revise it. Go back to the page atomic and ionic radii and read the section on ionic radii - but don't read the section headed "The relative sizes of ions and atoms", and which continues to the end of the page. You don't need it, and you risk getting seriously confused. | |||||||||||||||||||||||||||||||||||||
|
Note: In fact CIE have asked about the relative sizes of atoms and ions in June 2012 paper 23 Q1(b). They asked you to compare the sizes of sodium, magnesium and aluminium ions with the sizes of the original atoms. They said that the ions are smaller because the nuclei have a greater attraction because they are attracting fewer electrons, and also accepted the removal of a complete layer of electrons. The sizes of the negative ions that they were asking about relative to the atoms are much more difficult to explain satisfactorily, for reasons that I have gone into at length at the bottom of the page you have just read. What they accepted for 2 marks was "anions contain more electrons than the corresponding atoms, or anions contain more electrons than they do protons; nucleus has a smaller attraction". So if they ask something similar in the future, that is what you tell them. | |||||||||||||||||||||||||||||||||||||
|
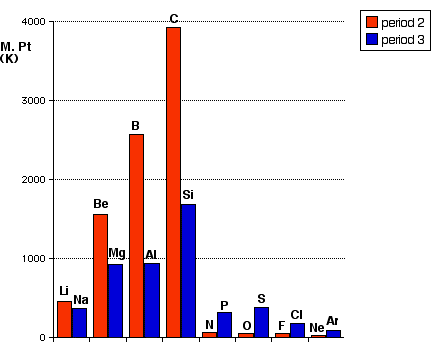
Melting points Then find the melting (and boiling) points discussed towards the bottom of the page atomic and physical properties of period 3 elements, but you should read the earlier section about the structures of the elements first. You can't understand the stuff about melting points unless you first understand the structures of the elements. Notice that I prefer the term "giant covalent" for structures like silicon, rather than "giant molecular" which CIE use. It would obviously make sense for CIE students to get used to the term you will meet in your exams. Melting points show good periodicity, in the sense that the pattern in Period 2 appears again in Period 3. This doesn't really fit in anywhere else on Chemguide, so here is an additional bar chart showing that:
You will see that the general pattern repeats in the two periods, although the detail doesn't. Both periods show the melting point increasing to Group 4, and then a steep drop. Although it isn't very difficult, explaining the differences between the two periods would actually take quite a long time, and is irrelevant to these statements (which are solely about Period 3) - so I am not going to confuse you with it. Electrical conductivity You may already have read this on the page atomic and physical properties of period 3 elements while you have been looking at some of the other things on this page. You will find it just above the section on melting and boiling points. A comment about October/November 2016 Paper 22 Q3(b)(ii) I am writing this particularly for teachers to warn them about a potentially misleading question and mark scheme. The question concerned the melting points of the elements in Period 3 which were shown graphically. The part of the period relevant to this discussion concerned sodium, magnesium and aluminium. The actual numbers for their melting points (not given in the question) are:
The previous question, Q3(b)(i), asked you to explain the increase from sodium to magnesium. That is a straightforward question about the factors affecting metallic bonding. The problem comes in the follow-up question which asked you to suggest why the increase between magnesium and aluminium is much smaller than that between sodium and magnesium. A "suggest" question means that you should be able to work out an answer from your previous knowledge. The acceptable responses in the mark scheme were all based on an assumption that the increase in the strength of the metallic bond as you go from magnesium to aluminium was much less than the increase between sodium and magnesium. The only problem with this is that it isn't true! If you repeat the above table, but this time include boiling points as well as melting points:
The metallic bond is only fully broken when the metal boils. The data shows that the metallic bond in aluminium is much stronger than in magnesium - the change from magnesium to aluminium is greater than that between sodium and magnesium. And if you want further convincing, we can add atomisation enthalpies to the table - the amount of energy needed to turn the solid metal into its gaseous atoms. That's exactly what you are doing if you are fully breaking the metallic bond.
So . . . the strength of the metallic bond increases from sodium to magnesium, and increases more from magnesium to aluminium. You cannot deduce that by looking only at the melting points. Melting point is not a reliable guide to the strength of a metallic bond. Why am I spending so long on a single one mark question? Because there is a real danger of teachers reading this mark scheme, assuming it is correct, and then teaching something which is just plain wrong. With the internet, it doesn't take long for this sort of misleading stuff to get everywhere. I have no idea why the increase in melting point from magnesium to aluminium is so small, and have pointed this out as long ago as 2005 on the page you will have read above. In that time, nobody has offered me a proper explanation, despite the probability that it will have been read by huge numbers of people. I have written to CIE about this, but the answer I received completely ignored my reservations about the question and its mark scheme.
© Jim Clark 2020 |
|||||||||||||||||||||||||||||||||||||