|
Chemguide: Support for CIE A level Chemistry Learning outcome 37: Analytical techniques 37.4 Proton (1H) NMR spectroscopy Learning outcomes 37.4.1, 37.4.2, 37.4.3, 37.4.4 and 37.4.5 These statements are about proton NMR spectra and their interpretation. You should read the statements in your syllabus, and then check them off when you have read the pages below. The order in the syllabus doesn't follow the order in the pages you will read. Start by reading the Chemguide page about the background to NMR spectroscopy. If you have already done carbon-13 NMR, a lot of the page will be familiar. Judging by past CIE papers, the most important things you need to know from this page are:
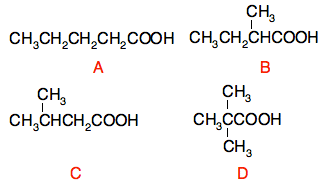
Now read the page about low resolution NMR spectra. CIE rarely (if at all?) ask questions about low resolution NMR spectra. You are reading this page as an introduction to the more difficult interpretation of high resolution spectra. You will be told values from an integration trace if they are relevant, and so you don't need to follow the link explaining how to work them out for yourself. Don't leave this page until you are sure that you understand how to get information from a low resolution NMR spectrum. Unless you are comfortable with this, you won't get far with the next page! You will be given a table of chemical shifts if you need them in an exam. You will find a copy of this table towards the bottom of the syllabus. Once you are confident, go on to the page about high resolution NMR spectra. Don't forget to look carefully at the effect of adding D2O to the substance, and the way this helps to identify an -OH group. It works the same way with N-H hydrogens in, say, an NH2 group. This is mentioned in statement 37.4.5 and is asked regularly in exams. Most CIE questions about NMR will expect you to get information about the structure from the way the lines split, and so you should be sure that you understand and can use the "n+1 rule". There is no suggestion in the syllabus or from past papers that you need to know the reasons for the n+1 rule. A comment about NMR and benzene rings This is a repeat of the same comment on the Chemguide page, but it is important that you don't worry unnecessarily about strange splitting patterns in the region of the spectrum containing hydrogens on benzene rings. At this level, all you can safely say about hydrogens attached to a benzene ring is how many of them there are. If you have a molecular formula which has 6 or more carbon atoms in it, then it could well contain a benzene ring. Look for NMR peaks in the 6.0 - 9.0 range. If you are given a number like 5 or 4 alongside that peak, this just tells you how many hydrogen atoms are attached to the ring. If there are 5 hydrogens attached to the ring, then there is only one group substituted into the ring. If there are 4 hydrogens attached, then there are two separate groups substituted in, and so on. There should always be a total of 6 things attached to the ring. Every hydrogen atom that is missing has been replaced by something else. Splitting patterns involving benzene rings are far too complicated for this level, and you shouldn't be expected to recognise or interpret them. A few extra thoughts arising from recent mark schemes and papers Information about peak areas In the past, questions have occasionally included numbers against each peak or cluster of peaks in a proton NMR spectrum. That number was a measure of the relative areas under the peaks, and that tells you about the relative numbers of hydrogens in the various environments. That's a really helpful bit of information. So, for example, if you had the numbers 3, 2 and 3 attached to each peak or set of peaks, that told you that the areas under the peaks were in the ratio 3:2:3, and so the hydrogens in those environments were also in the ratio 3:2:3. More recently they have frequently omitted those numbers, and then wanted you to predict the relative areas under the peaks, or the numbers of hydrogens (or protons) in each of the various environments. It is important to realise that it is impossible for you to get information about the relative areas from the spectrum you are given. There are no measurements you can take which will give it to you. Instead, you have to work back to this by working out what the structure of the molecule is, and then reporting the number of hydrogens (or protons) in each environment, and therefore the relative areas under the peaks. This can sometimes be quite tricky to do if you are trying to do it starting from scratch, but there may well be hints from earlier in the question. For example, November 2016 paper 42 Q8 was about the carboxylic acid isomers of C5H10O2. You were given the structure of one of them, and asked to draw the other three. You were later asked to identify which isomer gave a particular proton NMR spectrum. That involved identifying which group produced each peak or group of peaks, and stating the number of protons (hydrogens) in each environment. This is not so easy if you don't have any idea of the numbers of hydrogens in each environment to help you, but you do have information from the structures which you drew before. The four isomers are as follows (the letters A, B, C and D have nothing to do with the question, but give me a simple way to refer to them):
The spectrum showed four sets of peaks, which means that there must have been 4 different environments for the hydrogen atoms. Which of these structures has that? In A, the various CH2 groups are NOT in exactly the same environment. So there are 5 different environments here. (Don't forget the COOH hydrogen). In B, there are also 5 different environments - so that's no good. In C, there are two exactly equivalent CH3 groups - so those six hydrogens are all in exactly the same environment. That would give a total of 4 sets of peaks. That is definitely a possibility. In D, all the CH3 groups are exactly equivalent. This time there would only be 2 peaks in the spectrum (both singlets as it happens). Now that you know which structure you are talking about, you can look at the shift values and splitting patterns for the various peaks, and work out which is which. Then you can just count up the numbers of the various hydrogens and quote these as the relative areas under the peaks if you need to. The moral here is to look at ALL the information you have available in the question, and be flexible in how you approach it. That is easy to say, but hard to do under exam conditions. You really need to look at as many questions as you can, together with the mark schemes and Examiner's Report so that you know what to expect. Splitting patterns Where you have a peak made up of two sub-peaks, it is called a doublet NOT a duplet. If you use the word "duplet", you won't get any marks. Be careful how you explain splitting. You should use the phrase "It is a doublet because the neighbouring carbon atom(s) have one hydrogen." (and similar phrasing for triplets and quartets). That comes from an Examiner's Report complaining about lack of precision in what students were saying in an answer. If you come across the term "multiplet" rather than doublet, triplet or quartet, it is probably because there are more than 4 sub-peaks in the group. This will happen when the neighbouring carbon atoms have more than 3 hydrogens attached in total. The spectrum in the question we have just looked at had a multiplet for the CH carbon in structure C - there are a total of 8 hydrogens on the carbon atoms attached directly to it. Practise, practise, practise Interpreting high resolution NMR spectra needs a lot of practice. You should try to find as many questions as you can, starting with the most recent papers and working backwards. Look at the questions in association with the mark schemes, and if you find a question really difficult even with the mark scheme, look at the Examiner's Report for that exam as well. It may be that nobody could do it, in which case, you needn't worry about it.
© Jim Clark 2020 |