|
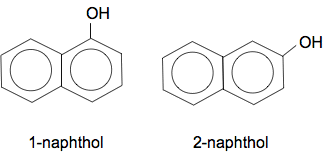
Chemguide: Support for CIE A level Chemistry Learning outcome 32: Hydroxy compounds 32.2: Phenol Learning outcomes 32.2.5, 32.2.6 and 32.2.7 These three statements are just odds and ends which in two cases just repeat previous material in this section. Statements 32.2.5 and 32.2.6 These statements are asking about why phenol is more reactive than benzene, and the directing effect of the OH group. Read the page about ring reactions of phenol again. Statement 32.2.7 This statement expects you to apply your knowledge of phenol's reactions to other phenolic compounds such as naphthol. This is new to this syllabus, and at the moment I have no idea of the level CIE will want. What follows is just a guess which I will change if necessary when there is more evidence. What is naphthol? Naphthol is based on naphthalene, which is a hydrocarbon where two benzene rings are fused together. In naphthol, an OH group has been attached to the one of the rings, in the same sort of way that phenol has an OH group attached to a simple benzene ring. There are two commonly discussed forms of naphthol depending on where the OH group is attached.
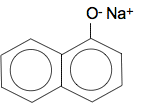
Naphthol reacts with sodium hydroxide solution Remember that phenol reacts with sodium hydroxide solution because phenol is slightly acidic. The hydrogen on the OH group is removed and you get sodium phenoxide formed. Naphthol behaves identically. For example, with 1-naphthol you get:
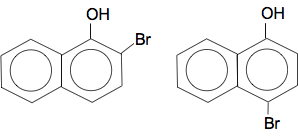
Which can be called sodium 1-naphthoxide. 2-naphthol would form exactly the same sort of of compound. Naphthol reacts with bromine water In principle this reacts the same as phenol. The OH group is 2,4-directing and activating - so the reaction works with bromine water with no need for pure bromine and a catalyst. If you substitute one bromine into 1-naphthol, you can get either of these compounds formed by substitution into either the 2- or 4-positions.
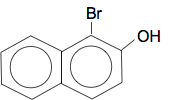
With 2-naphthol, the preferred place for the bromine to go is between the OH group and the other ring. There can't be any substitution in the 4-position, because that is cluttered up with the other ring.
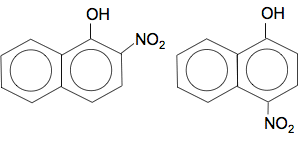
Multiple substitution is complicated here because you can get substitution into the other ring as well. I can't believe that even CIE would ask you about that without specifying it the syllabus. Naphthol reacts with nitric acid The position of substitution of the -NO2 group is exactly the same as the bromine case. For example, with 1-naphthol, you would get substitution into the 2- and 4-positions:
Naphthol reacts with diazonium salts to give azo compounds You will meet these reactions in statement 34.2.4, and we will leave it until then.
© Jim Clark 2020 |