|
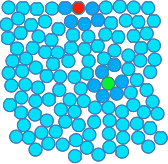
Chemguide: Support for CIE A level Chemistry Learning outcome 3.6 Intermolecular forces, electronegativity and bond properties These statements deal with the way molecules are attracted to each other. Before you go on, you should find and read the statements in your copy of the syllabus. You will find that I have changed the order of the statements compared with the way they appear in the syllabus, because the syllabus order doesn't seem logical to me. Don't worry about this - everything in this section will be covered. Statement 3.6.2 This is very much a repeat of learning outcome 3.1. Here are the important bits again: Electronegativity is one of the key concepts in chemistry, and so you should spend some time making sure that you fully understand it - what it means, what causes electronegativity differences, and what effects it has. To do that, first read the page about electronegativity in the bonding section of Chemguide. Read down as far as, but not including, the sections titled "Diagonal relationships in the Periodic Table" and "The polarising ability of positive ions". When you are sure that you understand the bits that you have read, follow the link at the bottom of that page to look at it all again in an organic chemistry context. Read that second page including the section on "Bond polarity and inductive effects". In outcome 3.1, I suggested that you left out that last bit, but it is necessary for this statement. Dipole moments This isn't mentioned anywhere in what you have read. Dipole moment is a measure of the separation of charge between the δ+ and δ- ends of a bond. It can also be used to measure the overall separation of charge on a polar molecule. You don't have to know how it is measured. All you need to know is that if there is a big separation of charge, the bond or molecule has a high dipole moment. If it has a small separation of charge, it has a low dipole moment. Statement 3.6.3(a) This statement deals introduces van der Waals forces. van der Waals forces are intermolecular forces - that is forces between molecules. You will find the term "intermolecular" discussed at the beginning of the first link below. You can wait until you get to it to read it. There are several different types of intermolecular force, and the term "van der Waals" covers all of them. You will meet the three most important ones as you read the rest of this page. Statement 3.6.3(b) van der Waals dispersion foces and permanent dipole-dipole interactions. The syllabus describes dispersion forces as instantaneous dipole - induced dipole (id - id) or London dispersion forces. They are commonly just called dispersion forces or London forces. The syllabus also talks about permanent dipole - permanent dipole (pd - pd) forces. Commonly these are just called dipole-dipole forces, implying the word "permanent". That is how you will find them described on the Chemguide page. If you are using the terms in an exam, then you should use the terms specified in the syllabus. In addition, this statement mentions hydrogen bonding as a form of dipole-dipole force. I am leaving discusion of this until after you have got the other terms sorted out.First, read the page about van der Waals forces. To reinforce this, look at the explanation of how van der Waals forces affect the halogens on a page about the physical properties of Group 7 (Group 17). You just need the section titled "Trends in Melting Point and Boiling Point" just over half-way down that page. Don't waste time at this stage looking at the rest of the page. Important: If you have read the follow-on page from the van der Waals page about the strengths of dispersion forces, you will know that dipole-dipole attractions contribute less to overall intermolecular attractions than dispersion forces do in the great majority of cases, and that dispersion forces can be quite strong. But a lot of textbooks and web sources still quote the traditional view that dispersion forces are the weakest of the intermolecular forces. What should you do if this comes up in an exam? This is almost certainly not something you need to worry about. CIE might, of course, expect you to know that a molecule which has dipole-dipole attractions as well as dispersion forces will probably have a boiling point greater than a similarly sized molecule with only dispersion forces. That is perfectly reasonable. Statements 3.6.1(a) and 3.6.3(c) These statements are about hydrogen bonding, and you should read them before you go on. Read this page on hydrogen bonding. You should note that the syllabus counts hydrogen bonding as a special case of van der Waals forces. Not all sources do this. Learn what CIE wants! Statement 3.6.1(b) This statement looks in more detail at the effect of hydrogen bonding on the properties of water. The relatively high boiling point of water compared with other molecules H2X, where X is one of the other elements in the same group as oxygen, was discussed at the beginning of the page on hydrogen bonding above. Its melting point, 0°C, is also far higher than the melting point of the next similar compound in the same group H2S, which melts at -85°C. If you were just talking about permanent dipole-dipole forces and dispersion forces, then the melting point of water would be less than that of H2S. Surface tension is just an effect of the strength of the forces between water molecules on the surface of the liquid. The simple diagram below represents water molecules in liquid water. I have coloured some of them for discussion purposes. The red one represents a molecule in the surface; the green one a molecule in the middle of the liquid. The molecules around these are darkened in colour just to pick them out.
In the middle of the liquid, there are hydrogen bonds between this molecule and all of the molecules surrounding it in all directions. On average, all those attractions will cancel out, and the green particle isn't going to be pulled in any particular direction over time. For the red molecule on the surface, there aren't any water molecules above it, and there will be a permanent net attraction downwards into the liquid by the particles underneath it. This is why you get a flat top surface which almost behaves as if it has a membrane of water molecules coverning it. This is the cause of surface tension. You will find more discussion on the effect of hydrogen bonding on the properties of water in the page on molecular structures. You don't need all of that page for the moment. Just read the two sections on "Ice" and "The unusual density behaviour of water" about 2/3 of the way down the page. Statement 3.6.4 I can't add anything to the syllabus statement - it is self-explanatory. Read it and make a note of it.
© Jim Clark 2019 |