|
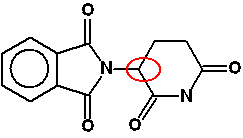
Chemguide: Support for CIE A level Chemistry Learning outcome 29: An introduction to A level organic chemistry 29.4: Isomerism: optical Learning outcome 29.4.4 This multi-part statement is about the importance of chirality in producing drugs. Chirality is the property of having non-superimposable mirror images. Statement 29.4.4(a) This statement talks about the potential differences in the biological activity of two enantiomers of a particular drug. Each chiral centre will have two possible arrangements of bonds around it. A different arrangement of bonds will mean a differently shaped molecule. And differently shaped molecules won't necessarily fit the active site of an enzyme, or whatever else the drug needs to attach to. A fairly simple example of a drug with a single chiral centre is thalidomide - a drug which was prescribed for morning sickness in pregnancy in the 1950s. The chiral centre is circled in red below:
This will have two optical isomers. One was the right shape and so did what it was supposed to, but the other one behaved completely differently, causing major birth defects. It is believed that the isomer responsible interfered with DNA. These days, a lot of effort goes into producing a single isomer of a drug. Chemical reactions frequently produce mixtures of optical isomers. Trying to produce just one isomer is known as asymmetric synthesis. Statement 29.4.4(b) This statement continues with this and suggests the need to separate a racemic mixture into two pure enantiomers. Many reactions involved in organic chemistry have mechanisms which inevitably produce a racemic mixture. A simple example involves the reaction between most aldehydes and HCN which you will have met in statement 17.1.3. You should re-read the page explaining the reaction, paying particular attention to the part about optical isomerism about 2/3 of the way down the page. This particular mechanism may or may not be involved in manufacturing a specific drug - it is just an example of how a particular reaction will automatically produce a racemic mixture. Reasons for producing drugs consisting of a single active isomer:
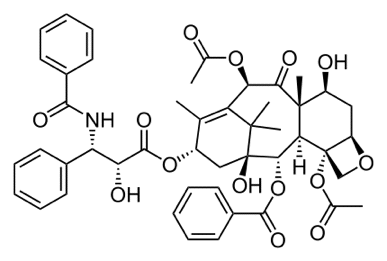
Statement 29.4.4(c) This statement is about the use of chiral catalysts to produce a single pure optical isomer. This is new to this syllabus, and until there are some exam questions, it isn't certain what CIE want. The easiest chiral catalyst to imagine is an enzyme, Enzymes are protein molecules which catalyse reactions in the cell. Read the first part of the Chemguide page about enzymes. You don't need to follow the link about protein structure, and you don't need to read beyond the heading "enzyme cofactors". And you don't need to learn any of this! What you do need is to understand that active sites on an enzyme will only hold molecules of a specific shape. So if an enzyme will process one optical isomer, it almost certainly won't process the other one because it probably won't fit. So biological systems tend to work with, and produce, only one of the possible enantiomers of a compound. Non-biological chiral catalysts work on a similar principle, but are much more difficult to describe in detail. There is a useful bit of video about chiral catalysts on YouTube. The most useful part starts at 3 minutes 07 seconds (3.07 on the time line) and lasts until 3 minutes 50 seconds (3.50 on the time line). You will most likely need to keep pausing the clip because it goes too fast to take in. Please don't think you have to learn this! The comment in brackets at the end of this statement Here is the structure of Taxol, an anti-cancer drug, shown as a skeletal formula. The Taxol molecule has a lot of chiral centres. These are carbon atoms which have four different groups attached to them. You can spot them very easily in this particular diagram of the structure of Taxol, because these are the ones which show the spatial arrangement of the atoms using either dotted or solid wedges.
Do NOT try to learn this - it is just an example that I already had from a previous syllabus! It is perfectly possible for a molecule to have more than one chiral centre. In the past CIE have drawn complicated structures like this in multi-choice papers and asked how many chiral centres there are. This one has 11 - make sure that you can get them all. It is much more difficult to do if you just have a simple skeletal formula. So look at each of the chiral centres in this molecule and make sure that you can see why each of the carbons has 4 different groups attached. If you find this difficult, re-read the page about optical isomerism, especially the part towards the bottom about "identifying chiral centres in skeletal formulae".
© Jim Clark 2020 |