|
Chemguide: Support for CIE A level Chemistry Learning outcome 28: Chemistry of transition elements 28.3: Colour of complexes Learning outcomes 28.3.1, 28.3.2, 28.3.3, 28.3.4 and 28.3.5 These statements are about the colours of transition metal complexes. Before you go on, you should find and read the statements in your copy of the syllabus. Basic material First read the page about the colours of complex metal ions. This will give you almost all of what you need for CIE purposes. About half-way down that page you will find a link to a further page which talks about the shapes of d orbitals and how this affects the way the energy levels of the d orbitals change when ligands are attached. It is essential that you follow this link because it covers what you need to know for statement 28.3.2. In case you have missed it, here is the link to the more about d orbitals page again. You should, however, read this at the point that it is placed on the main page. It won't make sense unless you have read the first part of that page first. CIE will ask about the splitting of the d-orbitals in tetrahedral complexes as well as the octahedral ones I concentrate on in the pages you have read. There is a description of what happens with tetrahedral complexes on the first page, but no diagram. The diagram will be exactly the same as for the octahedral splitting a bit further up the page except that the set of three orbitals now have a higher energy than the set of two. I suggest that it would be useful for you to draw that modified diagram for your notes. It will help you to remember it. Because you don't have to know the shapes of all the d-orbitals, it is hard to see how you could be asked a question explaining why the split happens in the way it does. All you will need is the fact that the d-orbitals split, and the splitting is different in octahedral and tetrahedral complexes. Don't forget to notice the use of the word "degenerate" referring to the d-orbitals which have the same energy before they are split by ligands attaching to the transition metal. On the pages you have read, I haven't use the term "non-degenerate" referring to the two sets of d-orbitals after splitting happens. You need to know what both of these terms mean for statement 28.3.1. Additional material for CIE A question over statement 28.3.5 Refer back to the page about the colours of complex metal ions. About 3/4 of the way down the page you will find a list of ligands in order of how much splitting they cause. You will see that ammonia produces more splitting than water, and water produces more splitting than hydroxide ions, and hydroxide ions more than chloride ions. The syllabus statement mentions these complexes with copper(II) ions and cobalt(II) ions. You have already met these in statement 28.2.7. The example which everyone concentrates on is the major colour difference in copper chemistry between the pale blue (cyan) aqua complex, and the very dark blue complex with ammonia - and this is discussed on that page. The complex containing OH groups is more of a problem. This is the neutral complex that you get when you add hydroxide ions to [Cu(H2O)6]2+ ions. Its formula is Cu(H2O)4(OH)2. Most of the ligands here, of course, are still water, and there isn't much difference between the colour of the precipitate and that of the original hexaaquacopper(II) ion solution. I'm not sure exactly what CIE want here. Up to November 2019, nothing specific had been set on it. CIE's questions about colour CIE's questions about the origin of colour in transition metal compounds have been straightforward recently (from 2016 - 2019). Typically they might ask you why a compound is coloured, or why two compounds have different colours.
In the second case, a typical mark scheme would be looking for the following points:
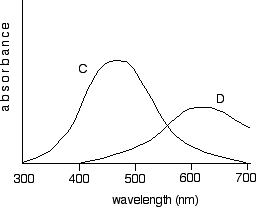
Obviously if the question was only asking about the origin of colour in a single compound, you wouldn't need the last point. Some questions need you to know the fact that the colour observed is the complement of the one absorbed. That means that you should learn the colour wheel shown on the Chemguide page. However, there have been questions which couldn't be answered by working out the complementary colour, because you were given examples where the light absorbed covered a wide range of frequencies. These were given as an absorption spectrum - something not on the syllabus. These questions aren't always difficult, but they have been difficult on occasions. Here is an example of a confusing one from a fairly old paper. The question gave you a graph like this for the absorption spectra of solutions of two transition metal complexes, C and D:
Absorbance is a measure of the amount of each wavelength being absorbed. The higher the point on the curve for a given wavelength, the more of that wavelength is being absorbed. What the question wanted to know was what colour the two solutions were likely to be. Your choices were yellow, red, green or blue. To help you, they included the following table:
Look at the graph labelled C. C is absorbing very strongly through most of the visible region of the spectrum. Although the spectrum peaks at somewhere between 450 - 500 nm, which is in the blue-green region, it is also absorbing a reasonable amount in the violet and yellow as well. Because it is absorbing light over such a wide range of colours, trying to think in terms of complementary colours doesn't really work. In this sort of case, you need to look at what wavelengths are actually getting through. In this case, there is least absorption in the red region of the visible spectrum. You are actually helped in this question by being given a small number of colours to choose between. One of those colours is red. And that's the right answer. Now try to work out what colour D is likely to be before you read on. D is absorbing strongly in the red and yellow regions, and least strongly in the violet and blue. Given the colour choices you have got, the only possible answer is blue. The other bit of this question asked which of C and D would have the higher energy gap between the two groups of d orbitals? This needs some careful thought. You have a choice between a complex which is absorbing most strongly in the red, or one absorbing most strongly in the blue-green area. The table tells you that the energy of a photon of light is higher for blue or green light than for red. The energy absorbed is due to the energy gap between the two groups of d orbitals. Energy is taken out of the light to promote an electron from the lower group to a space in the higher one. The energy needed to absorb blue or green light is higher than the energy needed to absorb red light. So the gap must be bigger in the one which absorbs blue or green light - in other words, in C. Please don't panic about this! Most recent questions have been much more straightforward. And if you do get a question like this, the truth is most people won't be able to do it - it will just cause the grade boundaries on that paper to fall. Obviously, if you can do it, that's a bonus. The relationships between wavelengths, frequency and energy You were helped in this particular question by being given the trend in energy over a set of wavelengths. That won't necessarily be the case. You will probably be given the information you need, but you can't be sure of that.
© Jim Clark 2020 |
|||||||||||||||||||