|
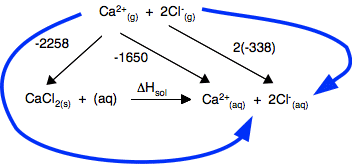
Chemguide: Support for CIE A level Chemistry Learning outcome 23: Chemical energetics 23.2: Enthalpies of solution and hydration Learning outcomes 23.2.1, 23.2.2, 23.2.3 and 23.2.4 Before you go on, find and read the statements in your syllabus. You will find what you need for these statements on the page Enthalpies of solution and hydration. There is one potentially confusing point in the example calculation I have used on that page where I have chosen to use a value of lattice dissociation energy in the diagram - in other words, a value with a positive sign. The CIE syllabus uses lattice formation energy, with a negative sign - but they just call it lattice energy. My choice here is because we normally think about the energetics of this in two stages. You need to put energy in to break up the crystal (a positive value) and then you get energy out when the ions hydrate (a negative value). If you are going to do it this way and CIE gives you a negative value for lattice energy, remember that is for making the lattice. You need to put heat in to break it, and so would change the negative into a positive sign. Alternatively you could use the negative value, reversing the arrow from the gaseous ions to the crystal. You would then have to find a different route around the diagram so that you didn't go against the flow of any arrow.
If you do the calculation, you will get exactly the same answer as on the page you have just read. This is probably safer, because it more closely reflects what CIE might ask.
© Jim Clark 2020 |