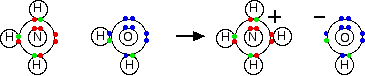|
Chemguide: Support for CIE A level Chemistry Learning outcome 12.1.2 Nitrogen and sulfur Learning outcome 12.1.2 This statement deals with the formation and structure of the ammonium ion, and its reactions with bases. Before you go on, you should find and read the statement in your copy of the syllabus. Statements 12.1.2(a) and 12.1.2(b) Ammonia is a typical weak base. It is a Bronsted-Lowry base - a substance which can accept hydrogen ions. If you aren't familiar with this, you might want to look at the page about theories of acids and bases. You don't need to know about Lewis acids or bases. Ammonia is a base because of the active lone pair on the nitrogen which can accept hydrogen ions. It reacts with water by removing a hydrogen ion from a water molecule to produce ammonium ions and hydroxide ions. A co-ordinate (dative covalent) bond is formed between the nitrogen and the incoming hydrogen ion.
However, the reaction is reversible, and at any one time about 99% of the ammonia is still present as ammonia molecules. Only about 1% has actually produced hydroxide ions.
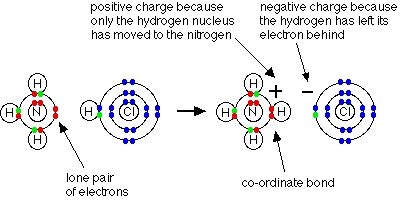
Ammonia can, of course, react with other more obvious acids to form ammonium salts. For example, ammonia reacts with hydrogen chloride gas in an acid-base reaction:
When the ammonium ion, NH4+, is formed, the fourth hydrogen is attached by a dative covalent bond, because only the hydrogen's nucleus is transferred from the chlorine to the nitrogen. The hydrogen's electron is left behind on the chlorine to form a negative chloride ion. Once the ammonium ion has been formed it is impossible to tell any difference between the dative covalent and the ordinary covalent bonds. Although the electrons are shown differently in the diagram, there is no difference between them in reality. Because the nitrogen atom has four bonding pairs arranged around it, and no lone pairs, its shape is tetrahedral - just like methane. Statement 12.1.2(c) Ammonia is only a weak base, and the extra hydrogen ion is easily removed again. Ammonium salts react readily with bases to produce ammonia gas. For example, ammonia is often prepared in the lab by heating an ammonium salt (such as ammonium chloride or ammonium sulfate) with a base such as calcium oxide or calcium hydroxide. For example:
This sort of reaction is also used as a test for ammonium ions in a compound. The solid compound (or its solution) is warmed with sodium hydroxide solution. Ammonia gas is produced which (apart from its smell) is recognised because it turns red litmus paper blue. The ionic equation for this reaction is:
Ammonium salts such as ammonium sulfate or ammonium nitrate are commonly used as fertilisers. CIE have on more than one occasion asked why a farmer wouldn't treat a field with an ammonium compound at the same time as using an alkali (a soluble base) like lime. The reason is obvious if you think about it. The ammonium salt will react with the base to give off ammonia which will escape into the atmosphere as a gas, and so not be available to the plants.
© Jim Clark 2020 |