|
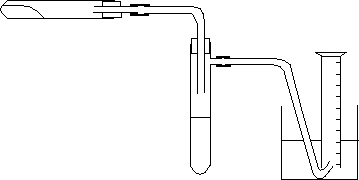
Chemguide: Support for CIE A level Chemistry Paper 5 Drawing diagrams for Paper 5 Drawing We will look at drawing diagrams first, and worry about the labelling later. Draw diagrams with a sharp HB pencil, and have a clean rubber available. Don't try to draw diagrams using a pen - you have to be able to rub out mistakes. The diagrams below suggest two possible solutions to a problem in which a solid is going to be heated in the horizontal tube. The solid produces another solid (which obviously remains in the tube) and two gases, both of which you have to measure in order to calculate how many moles of each are produced. You are told that one of the gases reacts with something which you can put in the side-armed boiling tube and so doesn't get any further through the apparatus. The other gas is collected in the gas syringe or over water in an inverted measuring cylinder. The diagrams show the apparatus before heating is started. All I want to concentrate on for now are the diagrams themselves. We will look at how you might design this piece of apparatus on a separate page.
The convention is that chemistry diagrams should be a cross-section through the apparatus - not a picture of the apparatus.
In the diagrams above, both of these are true. The gas mixture passes out of the horizontal tube, and into the liquid in the side-armed boiling tube. One of the gases reacts with that (which is what you want) and the other will bubble through into the gas syringe or measuring cylinder. How can you throw away marks in drawing these diagrams? Here is another version of one of the diagrams. Why wouldn't this work?
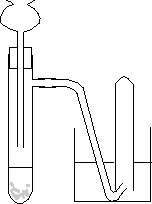
Because the tube in the side-armed boiling tube doesn't go below the surface of the liquid, most of the gas you are trying to trap here will just go straight on into the water and measuring cylinder. Here is a similar example in an experiment to collect a gas from the reaction between a solid and a liquid:
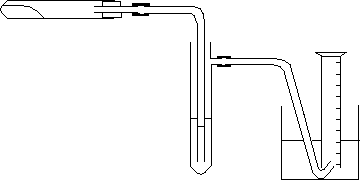
Inexperienced students will quite commonly draw this without thinking about it. Because the bottom of the thistle funnel isn't under the liquid, all of any gas produced will just escape through the thistle funnel. It isn't going to force its way out through the water into the collecting tube. You must look at your diagrams critically for any flaws! Back to one of the original diagrams again. What is wrong with this version?
It isn't uncommon for people to forget to draw one of the bungs! You would trap out the gas reacting with the liquid OK, but the other gas will just escape through the open top of the tube. That would lose you the mark for a workable experiment. And another faulty version of the same diagram. What is wrong this time?
It isn't a proper cross-section. Because of the way the bungs are drawn, the gas doesn't have a barrier-free way through the apparatus. Would CIE penalise you for this? I don't know - but it isn't worth taking the chance. Every mark you waste could be enough to drop you through a grade boundary so that your overall result isn't good enough for what you want to do. Labelling Diagrams should be labelled in ink, but don't overdo the labelling. It isn't necessary to label every single bung or bit of glass or rubber tubing. What you should do is label anything which isn't totally obvious.
| |
|
Note: I'll talk some more about circuit diagrams in discussing a tricky past question in the page about designing your experiment. | |
© Jim Clark 2017 |
|