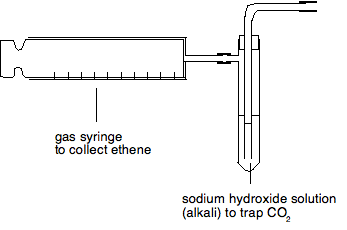|
Chemguide: Support for CIE A level Chemistry Paper 5 Choosing sensible apparatus in design questions Introduction One of the Paper 5 questions will ask you to design an experiment for some purpose. The introduction to the question will give you a lot of useful information, and you will need to read it carefully for hints. CIE could ask about anything from the entire A level syllabus, and I can't possibly cover everything. What I will do is to look in some detail at three cases which might prove awkward if you haven't done or seen a lot of practical work. Example 1 Experiments of this kind want you to add a solid to a liquid and then measure the volume of gas given off. This could be the total volume of gas given off, or a rate of reaction experiment where the volume of gas was measured during the course of the reaction, or perhaps the time taken to produce a certain volume of gas. It makes no difference to the practical set-up. The diagram below shows one possible way of doing this. It shows an experiment to measure the volume of carbon dioxide given off when dolomite (a magnesium limestone) reacts with dilute hydrochloric acid. It would work perfectly well with any other solid/liquid reaction which produced a gas.
We need to look at this in bits. Labelling Make sure you label everything which isn't obvious. There is no need to label things like bungs or bits of delivery tubing. All contents should be labelled, including the water in the beaker if you collect the gas over water. If in doubt, label it!Collecting the gas You could, of course, collect the gas over water into a measuring cylinder, but a gas syringe is simpler to sketch with less chance of making silly mistakes (like not putting the measuring cylinder over the end of the delivery tube, or not having the measuring cylinder full of water before you start, or drawing the delivery tube passing through the side of the beaker!). The reaction flask The diagram shows a simple and reliable way of mixing a weighed quantity of solid with the liquid without losing any gas in the process. There are other ways you could do it, but I am not giving them to avoid confusion. The liquid is measured into the flask first, and then the solid is lowered in using a weighing bottle on the end of a piece of cotton. Everything is set up, and when you are ready to start, you shake the flask, the weighing bottle falls over, and you continue shaking to make sure the solid and liquid are properly mixed. What you cannot do is just tip the solid into the flask and then quickly push the rubber bung in. You will lose gas before you get the bung in, and forcing the bung in will displace some air in the flask into the syringe. There are also problems using a thistle funnel to add the liquid to solid already in the flask. Use the method above. Other points Be precise about what measurements you are going to make. So, for example, you will need to find the mass of the weighing bottle and then the mass of the bottle plus the solid. If you are measuring volumes of gas, you will need to record the initial volume shown on the gas syringe, which may not necessarily be zero. It is possible that setting up the apparatus might force some air into the syringe. And of course you will need to record the final volume, or the volume after some measured time(s), depending on what the experiment is designed to do. And also be precise about observations. One CIE question asked how you would know that a reaction like this had stopped by only observing the gas collecting apparatus. The answer they wanted, of course, was "The volume in the syringe stops increasing." If you were collecting it over water, then you could say that there were no more bubbles passing into the measuring cylinder. An observation is something you actually see, not something you deduce. | |
|
Note: If students are asked what they observe if you add, say, magnesium to dilute hydrochloric acid, unless they have been properly trained, they will often say "Hydrogen is produced." But that isn't what you observe! You see bubbles of a colourless gas which you might test with a lighted splint. If it pops, you can deduce that it is hydrogen, but unless it came off waving little flags saying "hydrogen" you couldn't see that it was hydrogen. | |
|
Example 2 This is an example of having to collect and measure both gases in a mixture produced during a reaction. One CIE question involved heating anhydrous magnesium nitrate. It is fairly unlikely that CIE will use this particular example again, although there is a similar sort of problem (although even more complicated) in Example 3 below. What matters is that you don't make a fool of yourself if they come up with a different, but similar example. The question told you that heating magnesium nitrate produces magnesium oxide, nitrogen(IV) oxide and oxygen. You were also told that nitrogen(IV) oxide was an acidic gas which reacts readily and completely with alkalis. You were asked to write an equation for the reaction, and then design an experiment to confirm it. You would need to record the mass of the magnesium nitrate to start with so that you could work out how many moles of it you had, and then collect and measure the two gases separately so that you could work out how many moles of them were formed. The Examiner's Report was highly critical of some of the attempts to design a proper piece of apparatus. Some students were heating the magnesium nitrate in a container which had two tubes coming out of it, each leading to a gas syringe - one for the oxygen, the other for the nitrogen(IV) oxide. If you can't instantly see how stupid that is, stop and think! What exactly is going to persuade all the oxygen to go one way, and all the nitrogen(IV) oxide the other? You just have a random mixture of gases. A sensible solution is in the apparatus below.
If you pass the mixture of gases through an alkaline solution such as sodium hydroxide solution, the nitrogen dioxide will all react, and won't get any further through the apparatus. You can therefore measure the volume of oxygen collecting in the gas syringe. Knowing the volume and given some other information, it is possible to work out the number of moles of oxygen. This is going to be either a pV=nRT calculation or one based on the molar volume of a gas. What about the nitrogen(IV) oxide? You can't measure its volume, but you can measure its mass. If you weigh the tube containing the sodium hydroxide solution before and after the experiment, the increase in mass is due to the nitrogen(IV) oxide absorbed. If you know its mass, you can easily work out how many moles there are. | |
|
Note: You have to be able to do these basic mole calculations. Much of this question was based around this sort of sum. | |
|
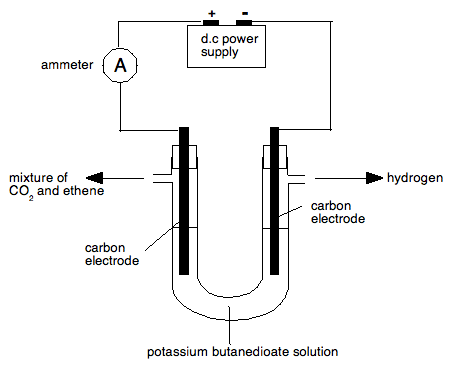
There is nothing at all sophisticated in any of this, but if you were faced with it in an exam for the first time, you could easily panic. Well, that shouldn't happen now! Example 3 This involves a complex bit of apparatus, which follows on from Example 2, but adds another problem. If you want to look it up it was in May/June 2015 paper 52. The questions concerned the electrolysis of potassium butanedioate solution, and you were given the equation. Three gases were produced. Hydrogen at one electrode, and a mixture of carbon dioxide and ethene at the other one - you weren't told which came off which electrode. You were also told that carbon dioxide could be removed by absorbing it in an alkali before the ethene was collected. This is just like Example 2 as far as the carbon dioxide and ethene is concerned. Your extra problem is keeping the gases produced at the two electrodes separate so that the hydrogen doesn't get mixed in with the carbon dioxide and ethene. There is a standard bit of apparatus that you can use to do this which most students should have come across, but if you haven't then you would have a bit of difficulty with this. You use a side-armed U-tube fitted with two carbon electrodes. There is then no possibility of the gases from the two electrodes mixing, and the side-arms can be connected easily to whatever you are going to use to collect or absorb the gases. Don't forget to draw the bungs, otherwise you won't collect anything. Instead you will throw away a mark!
Now let's put in the circuit diagram (which the question asked for) and some labelling. You need to measure the current (the question also asked for that) and so you need an ammeter. Some students apparently used a voltmeter, and so threw away a mark.
The symbol to be used for the power source was given in the question. The next thing to decide is which gases come off from which electrode.
So we have this:
Now you have to collect everything, separating the carbon dioxide and ethene as you do it. The hydrogen can simply be collected in a gas syringe. The ethene and carbon dioxide can be separated in exactly the same way as we separated the nitrogen(IV) oxide and oxygen in Example 2.
As a result of all this, you will have measured volumes of ethene and hydrogen, and you can find out how much carbon dioxide there is by weighing the side-arm boiling tube and its contents before and after the experiment. | |
|
Note: Sorry! There is no way that I can draw this all in one diagram on screen without making everything very small and confusing. Imagine that you had to do this on an exam paper, and draw the complete set-up so that it will fit (including labelling). You would have no more than about 18 cm x 12 cm. Do it now so that you can see exactly what you would have to do. | |
|
These examples were chosen to help you with particular problems. Make sure that this is all obvious to you, but don't expect to see exactly the same examples again. The chances of them coming up in exactly the same form in the future are probably close to zero. As with all exams, the best way of preparing for this is to do lots of past papers, looking carefully at mark schemes and Examiner's Reports. As far as possible, concentrate on the most recent papers, and don't forget that the syllabus changed in a major way for exams from 2016 onwards. Papers before that may contain material which is no longer on the syllabus (although most of the practically-based stuff won't have changed much).
© Jim Clark 2017 |
|