|
Chemguide: Support for CIE A level Chemistry Learning outcome 4.2 Bonding and structure This statement looks at the lattice structures of a number of solid crystals with various sorts of bonding. Before you go on, you should find and read the statement in your copy of the syllabus. Learning outcomes 4.2.1 and 4.2.2 I am taking these together because the Chemguide pages you will visit don't separate them. Statement 4.2.1(a) is about ionic structures such as sodium chloride and magnesium oxide. You will find this explained on the page about ionic structures. You can ignore the section about caesium chloride. You will find magnesium oxide mentioned in passing - all you need to know about it is that it has exactly the same crystal structure as sodium chloride, but that the forces holding the ions together are greater because 2+ is attracting 2-, rather than 1+ attracting 1- in the sodium chloride case. Statement 4.2.1(b) is about simple molecular crystal structures such as iodine, buckminsterfullerene (C60) and ice. You will find some of this on the page about molecular structures. Read down as far as iodine and ice. You don't need the bits about polymers for the moment. For iodine, it is probably enough to know that the crystal of iodine consists of iodine molecules packed closely together, and held in place by relatively weak van der Waals forces. I haven't been able to find any trace of a question dealing with the exact way the molecules pack. Buckminsterfullerene You almost certainly know about the two main allotropes of carbon - diamond and graphite. And you should also be able to define the word "allotrope". Allotropes are two or more different forms of an element in the same physical state. In a CIE question, they wanted you to go a bit further than this by saying that this was because the different allotropes had different arrangements of the atoms. So learn this:
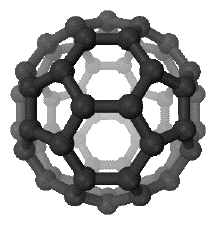
In 1985, a third allotrope of carbon was discovered, known as buckminsterfullerene or buckyballs. Buckminsterfullerene, C60, is a carbon molecule made of 60 atoms arranged like the faces of a soccer ball - in pentagons and hexagons. That is why it is commonly known as buckyballs. This is the simplest of a family of similar nanoparticles known as fullerenes. Nanoparticles usually have sizes in the range of 1 to 100 nm, where 1 nm is 10-9 metres. For comparison purposes, the covalent radius of a carbon atom is 0.077 nm.
| |
|
Note: This diagram was taken from Wikipedia. | |
Statement 4.2.1(c) is about giant molecular crystal structures such as silicon(IV) oxide (silicon dioxide) and the diamond and graphite allotropes of carbon. You will find all of this on the page about giant covalent structures. I personally prefer the term "giant covalent structure" to "giant molecular structure", although both terms are in common use. Strictly speaking a molecule contains a definite number of atoms joined together. That isn't true of these giant structures, where the number of atoms depends on the size of the crystal. Statement 4.2.1(d) is about metallic structures. You will find this on the page about metallic structures. Copper (mentioned in the syllabus) isn't specifically discussed, but it is a 12-co-ordinated metal, and so you should concentrate on the parts of the page related to 12-co-ordination. If you haven't done it recently, it might pay you to follow the link back to metallic bonding that you will find near the top of that page before you read on. Learning outcome 4.2.3 This statement asks you to be able to predict the sort of structure and bonding present in a substance from information about its properties You should find all that you will need on this Chemguide page.
© Jim Clark 2019 |
|