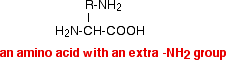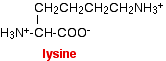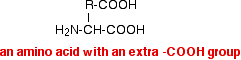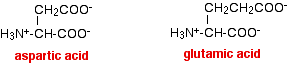|
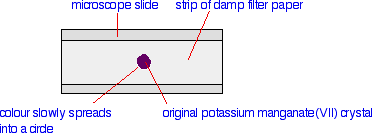
Chemguide: Support for CIE A level Chemistry Learning outcome 34: Nitrogen compounds 34.4: Amino acids Learning outcome 34.4.3 This statement is about electrophoresis and how different amino acids or small peptides behave during it. Before you go on, you should find and read the statement in your copy of the syllabus. The background to electrophoresis A simple inorganic example Suppose you put a single crystal of potassium manganate(VII) (potassium permanganate) onto some damp filter paper supported on a microscope slide. The crystal would dissolve in the water in the filter paper, and the deep purple colour would diffuse out to make a small circle around the original crystal.
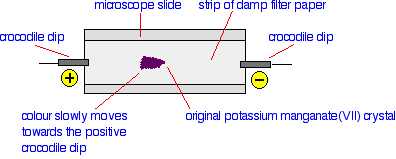
Now let's modify this by connecting the filter paper into a simple electric circuit. This time, when you put the potassium manganate(VII) crystal onto the paper, the colour doesn't spread into a circle. Instead, the purple colour starts to move towards the positively charged crocodile clip.
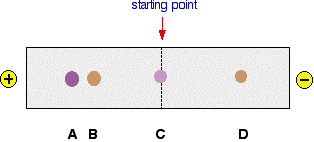
There isn't anything very surprising about this. The colour of the potassium manganate(VII) is due to the manganate(VII) ions present. These are negatively charged, MnO4-, and move towards the positive electrode. This movement of ions in an electric field is the basis of a separation technique known as electrophoresis. Let's apply this to the more interesting (and more complicated) case of amino acids. Electrophoresis involving amino acids Results of the electrophoresis of a mixture of simple amino acids In practice, paper isn't used for serious electrophoresis. Instead, a gel is used. Control of the pH is important, and so the gel is soaked in a buffer solution. Little troughs are made in the gel to hold the solutions being tested. In an exam, you may find that you have to interpret diagrams based on bits of paper or slabs of gel. It makes no difference whatsoever to what you would need to say. Unlike potassium manganate(VII), amino acids are colourless. To find out where they have got to, they are sprayed with ninhydrin which shows up the amino acids as brown or purplish stains. The diagram shows the effect of electrophoresis on a mixture of four amino acids at a known pH. This is just on a strip of paper, and the mixture was originally a single drop on the start line.
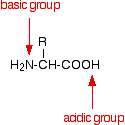
We need to explain why the four amino acids have moved in this way. Spot C The amino acid responsible for spot C hasn't moved. Why not? Remember that an amino acid has both a basic amine group and an acidic carboxylic acid group.
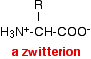
There is an internal transfer of a hydrogen ion from the -COOH group to the -NH2 group to leave an ion with both a negative charge and a positive charge. This is called a zwitterion.
At a particular pH, known as the isoelectric point, this is how the amino acid exists in solution. The amino acid won't move during electrophoresis, because the two charges cancel each other out, and there won't be any attraction either to the positive or the negative electrode. At any other pH, there will be some movement one way or the other - and we will explore that later. So amino acid C doesn't move because the pH of the solution happens to be exactly the same as the isoelectric point. | |
|
Note: For many simple amino acids, the isoelectric point is at a pH of about 6. I have, however, come across the odd exam question which gives a diagram a bit like the one above, but describes the solution as being neutral. This is a bit careless! In fact, an amino acid with an isoelectric point of 6 would in fact move slightly towards the positive electrode at pH 7. This is discussed in detail at the bottom of the page about the acid-base behaviour of amino acids. | |
|
Spot D This amino acid has moved towards the negative electrode, and so must be positively charged. This positive charge results from having an extra -NH2 group in the amino acid, like this:
In a buffer solution with a pH around neutral, this will be present as the ion:
The extra -NH2 group in the amino acid picks up a hydrogen ion from the water. Amino acids like this carry a net 1+ charge in a solution which is around neutral. The amino acid lysine is an example of this. You don't need to remember this formula (or the formulae of the other named amino acids mentioned below).
During electrophoresis at a pH about neutral, amino acids like this will travel towards the negative electrode. Spots A and B These travel towards the positive electrode and so must be negatively charged at this pH. This happens in amino acids which carry an extra -COOH group, for example:
In a buffer solution with a pH around neutral, this will be present as the ion:
The extra -COOH group loses a hydrogen ion to the water, to leave an ion which has an overall charge of 1-, and which therefore moves towards the positive electrode. Two examples of this are aspartic acid and glutamic acid.
The question remains as to why the two amino acids in our electrophoresis example have travelled different distances. One of the factors which affects this is the size of the ions. The ions have to find their way through the fibres in the paper or the pores in the gel. Smaller ions will travel faster than bigger ones. So, for example, the smaller aspartic acid will travel faster than the larger glutamic acid. What do I mean by "smaller" or "larger"? This could be in terms of the masses of the ions, or their shapes. Ions with bulky groups (such as benzene or other rings) will travel more slowly than ones with, say, unbranched chains. Since there are only two naturally occurring amino acids with an extra -COOH group, spot A must be due to aspartic acid and spot B to glutamic acid. Changing the pH of electrophoresis experiments with amino acids This should be fairly obvious if you understand about the acid-base behaviour of amino acids. What happens if the buffer solution you use has a low pH? In the presence of a sufficiently acidic solution, all the -COOH groups present will exist as -COOH groups, and not as ions. All the -NH2 groups will have picked up hydrogen ions to form -NH3+. That means that all of the amino acids will carry a positive charge, and all of them will move towards the negative electrode. The three different sorts of amino acid that we have been looking at will have ions like this at a low enough pH:
All of them will move to the negative electrode, but they will move at different rates because, for example, one of them carries 2+ charges whereas the others carry only 1+. And obviously they will be different sizes as well - both in mass and shape. What happens if the buffer solution you use has a high pH? In the presence of a sufficiently alkaline solution, all the -COOH groups present will exist as -COO- ions. All the -NH2 groups will exist as simple -NH2 groups - not ions. That means that all of the amino acids will carry a negative charge, and all of them will move towards the positive electrode. The three different sorts of amino acid that we have been looking at will have ions like this at a high enough pH:
All of them will move to the positive electrode, but they will move at different rates because, for example, one of them carries 2- charges whereas the others carry only 1-. And obviously they will be different sizes as well - both in mass and shape. What happens if the buffer solution you use has some intermediate pH - not very high or very low? CIE have asked questions using buffer solutions of various other values, and you need to understand how to handle questions like this. If they do this, they will give you figures for the isoelectric points for the various amino acids. Think it out like this:
The effect of charge and size on how far an amino acid moves during electrophoresis Look at the total number of charges at the pH of the experiment. An amino acid with, say, a total of 2- charges will move faster and further than one with only 1- charge. This might happen if the amino acid contains two COOH groups, and the pH is greater than the isoelectric point. Similarly one with 2+ charges will move faster and further than one with 1+ charge. This might happen if the amino acid contained two -NH2 groups, and the pH was lower than the isoelectric point. Smaller amino acids will move faster and further than bigger ones with the same overall charge. A bigger amino acid with two charges at the pH of the experiment may well move about the same distance as one half the size but with only one charge. So in an exam, remember:
Other factors affecting the movement of the amino acids Voltage matters. The higher the voltage, the greater the speed at which the amino acids move through the paper or gel. Temperature also matters. An increase in temperature can speed up electrophoresis. Electrophoresis involving peptides CIE have asked questions in which electrophoresis is carried out using a mixture of a dipeptide and a couple of amino acids. Here is a general structure for a dipeptide. R and R' can be the same or different.
If you look at this carefully, you will see that it is essentially just a bigger amino acid - except that this time the -NH2 and -COOH groups are at opposite ends ot the molecule rather than next door to each other. If there aren't any other -NH2 or -COOH groups in the R groups, then the isoelectric point of the dimer will be about 6. If there is an extra -NH2 group, then it will have an isoelectric point similar to a single amino acid containing an -NH2 group. CIE gave an example of this in a question in November 2018 paper 43 Q6 involving a dipeptide which included lysine and valine. They told you that the isoelectric point for lysine was 9.8. In those days, formulae for amino acids were included in the Data Booklet; nowadays they will give you the formula. Anyway, lysine has an extra -NH2 group. You could assume that the presence of lysine in the dipeptide would give it a similar isoelectric point. The same sort of argument applies if there is an extra -COOH group in an amino acid. That produces an isoelectric point of about pH 3. A dipeptide containing such an extra group would have a similar sort of isoelectric point.
© Jim Clark 2020 |
|